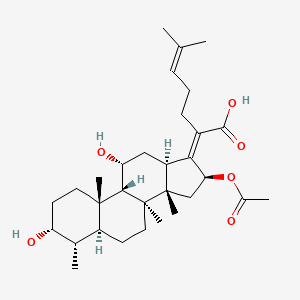

1. Acid, Fusidic
2. Fucithalmic
3. Fusidate, Silver
4. Fusidin
5. Silver Fusidate
6. Sodium Fusidate
7. Stanicide
1. Fusidine
2. 6990-06-3
3. Ramycin
4. Fucithalmic
5. Fusidate
6. Fucidic Acid
7. Fucidin Acid
8. Fucidin
9. Flucidin
10. Fucidate
11. Taksta
12. Sodium Fusidate
13. Sq 16,603
14. (-)-fusidic Acid
15. (2z)-2-[(3r,4s,5s,8s,9s,10s,11r,13r,14s,16s)-16-acetyloxy-3,11-dihydroxy-4,8,10,14-tetramethyl-2,3,4,5,6,7,9,11,12,13,15,16-dodecahydro-1h-cyclopenta[a]phenanthren-17-ylidene]-6-methylhept-5-enoic Acid
16. Anhydrous Fusidic Acid
17. Cem-102
18. Nsc-56192
19. Sq 16603
20. Mls001332649
21. Chebi:29013
22. 59xe10c19c
23. Smr000857101
24. Sq-16603
25. (2e)-2-[(3r,4s,5s,8s,10s,11r,13r,14s,16s)-16-acetyloxy-3,11-dihydroxy-4,8,10,14-tetramethyl-2,3,4,5,6,7,9,11,12,13,15,16-dodecahydro-1h-cyclopenta[a]phenanthren-17-ylidene]-6-methylhept-5-enoic Acid
26. Acide Fusidique
27. Acido Fusidico
28. Fusidinic Acid
29. Acidum Fusidicum
30. Mfcd00865135
31. (-)-16beta-acetoxy-3alpha,11alpha-dihydroxyfusida-17(20)z,24-diene-21-oic Acid
32. (2z)-2-[(3alpha,4alpha,5alpha,8alpha,9beta,11alpha,13alpha,14beta,16beta,17z)-16-(acetyloxy)-3,11-dihydroxy-4,8,10,14-tetramethylgonan-17-ylidene]-6-methylhept-5-enoic Acid
33. (2z)-2-[(3r,4s,5s,8s,9s,10s,11r,13r,14s,16s)-16-acetoxy-3,11-dihydroxy-4,8,10,14-tetramethyl-2,3,4,5,6,7,9,11,12,13,15,16-dodecahydro-1h-cyclopenta[a]phenanthren-17-ylidene]-6-methyl-hept-5-enoic Acid
34. (3alpha,4alpha,8alpha,9beta,11alpha,13alpha,14beta,16beta,17z)-16-(acetyloxy)-3,11-dihydroxy-29-nordammara-17(20),24-dien-21-oic Acid
35. (z)-2-((3r,4s,5s,8s,9s,10s,11r,13r,14s,16s)-16-acetoxy-3,11-dihydroxy-4,8,10,14-tetramethyldodecahydro-1h-cyclopenta[a]phenanthren-17(2h,10h,14h)-ylidene)-6-methylhept-5-enoic Acid
36. Fusidic Acid (usan/inn)
37. Fusidic-acid
38. Fusidate Acid
39. 1qca
40. Fusidicacid
41. C.a.s. 62,602
42. Prestwick2_000390
43. Fusidic Acid [mi]
44. Fusidic Acid [inn]
45. Fusidic Acid [usan]
46. Schembl25646
47. Mls001332650
48. Mls002207094
49. Unii-59xe10c19c
50. Acide Fusidique [inn-french]
51. Acido Fusidico [inn-spanish]
52. Acidum Fusidicum [inn-latin]
53. Fusidic Acid [who-dd]
54. Cem102
55. Chembl374975
56. Dtxsid0023086
57. Fusidic Acid [usan:inn:ban]
58. Bdbm58924
59. Cid_3000226
60. Gtpl10815
61. Hms2235b11
62. Fusidic Acid [ep Impurity]
63. Act03304
64. Ex-a3797
65. Fusidic Acid [ep Monograph]
66. Hy-b1350
67. Nsc56192
68. Zinc8143796
69. Einecs 230-256-0
70. Fusidic Acid For Peak Identification
71. Nsc 56192
72. S3971
73. 16-(acetyloxy)-3,11-dihydroxy-29-nordammara-17(20),24-dien-21-oic Acid
74. Akos005146257
75. Ccg-269829
76. Db02703
77. Ds-3261
78. Lmpr0106040001
79. Ncgc00485232-01
80. (2z)-2-[(17z)-16beta-acetoxy-3alpha,11alpha-dihydroxy-4alpha,8alpha,10,14beta-tetramethyl-5alpha,9beta,13alpha-gonan-17-ylidene]-6-methylhept-5-enoic Acid
81. 29-nordammara-17(20),24-dien-21-oic Acid, 16-(acetyloxy)-3,11-dihydroxy-, (3alpha,4alpha,8alpha,9beta,11alpha,13alpha,14beta,16beta,17z)-
82. 3.alpha.,11.alpha.,16.beta.-trihydroxy-29-nor-8.alpha.,9.beta.,13.alpha.,14.beta.-dammara-17(20),24-dien-21-oic Acid 16-acetate
83. C.a.s. 62,602; Diethanolamine Fusidate
84. Cs-0013095
85. F1007
86. C06694
87. D04281
88. 990f063
89. Q259930
90. Q-201141
91. Fusidic Acid, European Pharmacopoeia (ep) Reference Standard
92. Fusidic Acid For Peak Identification, European Pharmacopoeia (ep) Reference Standard
93. (2z)-2-[(3beta,4beta,5alpha,8alpha,9beta,11beta,13alpha,16beta,17z)-16-(acetyloxy)-3,11-dihydroxy-4,8,10,14-tetramethylgonan-17-ylidene]-6-methylhept-5-enoic Acid
94. (2z)-2-[(3r,4s,5s,8s,9s,10s,11r,13r,14s,16s)-16-acetyloxy-3,11-dihydroxy-4,8,10,14-tetramethyl-2,3,4,5,6,7,9,11,12,13,15,16-dodecahydro-1h-cyclopenta[a]phenanthren-17-ylidene]-6-methyl-5-heptenoic Acid
95. (2z)-2-[(3r,4s,5s,8s,9s,10s,11r,13r,14s,16s)-16-acetyloxy-4,8,10,14-tetramethyl-3,11-bis(oxidanyl)-2,3,4,5,6,7,9,11,12,13,15,16-dodecahydro-1h-cyclopenta[a]phenanthren-17-ylidene]-6-methyl-hept-5-enoic Acid
96. (3alpha,4alpha,8alpha,9beta,11alpha,13alpha,147beta,167beta,17z)-16-(acetyloxy)-3,11-dihydroxy-29-nordammara-17(20),24-dien-21-oic Acid
97. 29-nor-8.alpha.,13.alpha.,14.beta.-dammara-17(20),24-dien-21-oic Acid, 3.alpha.,11.alpha.,16.beta.-trihydroxy-, 16-acetate, (z)-
98. 29-nor-8alpha,9beta,13alpha,14beta-dammara-17(20),24-dien-21-oic Acid, 3alpha,11alpha,16beta-trihydroxy-, 16-acetate, (z)-
99. 29-nordammara-17(20), 16-(acetyloxy)-3,11-dihydroxy-, (3.alpha.,4.alpha.,8.alpha.,9.beta.,11.alpha.,13.alpha.,14.beta.,16.beta.,17z)-
100. 29-nordammara-17(20),24-dien-21-oic Acid, 16-(acetyloxy)-3,11-dihydroxy-, (3.alpha.,4.alpha.,8.alpha.,9.beta.,11.alpha.,13.alpha.,14.beta.,16.beta.,17z)
101. 29-nordammara-17(20),24-dien-21-oic Acid, 16-(acetyloxy)-3,11-dihydroxy-, (3a,4a,8a,9b,11a,13a,14b,16b,17z)-
102. 3.alpha.,16.beta.-trihydroxy-29-nor-8.alpha.,9.beta.,13.alpha.,14.beta.-dammara-17(20),24-dien-21-oic Acid 16-acetate
103. 3alpha,11alpha,16beta-trihydroxy-29-nor-8alpha,9beta,13alpha,14beta-dammara-17(20),24-dien-21-oic Acid 16-acetate
| Molecular Weight | 516.7 g/mol |
|---|---|
| Molecular Formula | C31H48O6 |
| XLogP3 | 5.5 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 6 |
| Exact Mass | 516.34508925 g/mol |
| Monoisotopic Mass | 516.34508925 g/mol |
| Topological Polar Surface Area | 104 Ų |
| Heavy Atom Count | 37 |
| Formal Charge | 0 |
| Complexity | 994 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 10 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the treatment of bacterial infections.
Fusidic acid is a bacteriostatic antibiotic and helps prevent bacterial growth while the immune system clears the infection.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
Protein Synthesis Inhibitors
Compounds which inhibit the synthesis of proteins. They are usually ANTI-BACTERIAL AGENTS or toxins. Mechanism of the action of inhibition includes the interruption of peptide-chain elongation, the blocking the A site of ribosomes, the misreading of the genetic code or the prevention of the attachment of oligosaccharide side chains to glycoproteins. (See all compounds classified as Protein Synthesis Inhibitors.)
D - Dermatologicals
D06 - Antibiotics and chemotherapeutics for dermatological use
D06A - Antibiotics for topical use
D06AX - Other antibiotics for topical use
D06AX01 - Fusidic acid
D - Dermatologicals
D09 - Medicated dressings
D09A - Medicated dressings
D09AA - Medicated dressings with antiinfectives
D09AA02 - Fusidic acid
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01X - Other antibacterials
J01XC - Steroid antibacterials
J01XC01 - Fusidic acid
S - Sensory organs
S01 - Ophthalmologicals
S01A - Antiinfectives
S01AA - Antibiotics
S01AA13 - Fusidic acid
Absorption
Sodium fusidic acid tablets have a 91% oral bioavailability. Absorption of the film-coated tablets is complete when compared to a solution, however oral absorption is variable. Oral fusidic acid hemihydrate (suspension) achieved a 22.5% bioavailability in pediatric patients following a 20 milligram/kilogram dose.
Metabolites include dicarboxylic ester/acid, 3-keto fusidic acid, hydroxy fusidic acid, glucuronide fusidic acid and a glycol metabolite.
Approximately 5 to 6 hours in adults.
Fusidic acid works by interfering with bacterial protein synthesis, specifically by preventing the translocation of the elongation factor G (EF-G) from the ribosome. It also can inhibit chloramphenicol acetyltransferase enzymes.

LOOKING FOR A SUPPLIER?