
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Australia

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
API
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
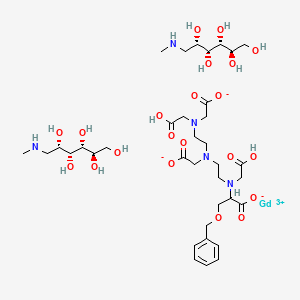

1. 3,6,9-triaza-12-oxa-3,6,9-tricarboxymethylene-10-carboxy-13-phenyltridecanoic Acid, Gadolinium
2. B 19036
3. B-19036
4. Gadobenate Dimeglumine
5. Gadobenic Acid
6. Gadobenic Acid, Dimeglumine Salt
7. Gadolinium-benzyloxypropionyl Tetraacetate
8. Gadolinium-bopta-dimeg
9. Gd(bopta)2
10. Gd-bopta
1. Gadobenate Dimeglumine
2. Gd-bopta/dimeg
3. Gadobenic Acid Dimeglumine Salt
4. B-19036/7
5. E-7155
6. Q21080030
| Molecular Weight | 1058.1 g/mol |
|---|---|
| Molecular Formula | C36H62GdN5O21 |
| Hydrogen Bond Donor Count | 14 |
| Hydrogen Bond Acceptor Count | 26 |
| Rotatable Bond Count | 29 |
| Exact Mass | 1058.31784 g/mol |
| Monoisotopic Mass | 1058.31784 g/mol |
| Topological Polar Surface Area | 440 Ų |
| Heavy Atom Count | 63 |
| Formal Charge | 0 |
| Complexity | 860 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 8 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 4 |
| 1 of 4 | |
|---|---|
| Drug Name | Multihance |
| PubMed Health | Gadobenate (Intravenous route) |
| Drug Classes | Radiological Ionic Contrast Media |
| Drug Label | MultiHance injection is supplied as a sterile, nonpyrogenic, clear, colorless to slightly yellow, aqueous solution intended for intravenous use only. Each mL of MultiHance contains 529 mg gadobenate dimeglumine and water for injection. MultiHance con... |
| Active Ingredient | Gadobenate dimeglumine |
| Dosage Form | Injectable |
| Route | Intravenous |
| Strength | 7.935gm/15ml (529mg/ml); 10.58gm/20ml (529mg/ml); 5.29gm/10ml (529mg/ml); 2.645gm/5ml (529mg/ml) |
| Market Status | Prescription |
| Company | Bracco |
| 2 of 4 | |
|---|---|
| Drug Name | Multihance multipack |
| Active Ingredient | Gadobenate dimeglumine |
| Dosage Form | Injectable |
| Route | Intravenous |
| Strength | 52.9gm/100ml (529mg/ml); 26.45gm/50ml (529mg/ml) |
| Market Status | Prescription |
| Company | Bracco |
| 3 of 4 | |
|---|---|
| Drug Name | Multihance |
| PubMed Health | Gadobenate (Intravenous route) |
| Drug Classes | Radiological Ionic Contrast Media |
| Drug Label | MultiHance injection is supplied as a sterile, nonpyrogenic, clear, colorless to slightly yellow, aqueous solution intended for intravenous use only. Each mL of MultiHance contains 529 mg gadobenate dimeglumine and water for injection. MultiHance con... |
| Active Ingredient | Gadobenate dimeglumine |
| Dosage Form | Injectable |
| Route | Intravenous |
| Strength | 7.935gm/15ml (529mg/ml); 10.58gm/20ml (529mg/ml); 5.29gm/10ml (529mg/ml); 2.645gm/5ml (529mg/ml) |
| Market Status | Prescription |
| Company | Bracco |
| 4 of 4 | |
|---|---|
| Drug Name | Multihance multipack |
| Active Ingredient | Gadobenate dimeglumine |
| Dosage Form | Injectable |
| Route | Intravenous |
| Strength | 52.9gm/100ml (529mg/ml); 26.45gm/50ml (529mg/ml) |
| Market Status | Prescription |
| Company | Bracco |
Contrast Media
National Library of Medicine's Medical Subject Headings. Gadobenic Acid. Online file (MeSH, 2014). Available from, as of October 23, 2014: https://www.nlm.nih.gov/mesh/2014/mesh_browser/MBrowser.html
MultiHance is indicated for intravenous use in magnetic resonance imaging (MRI) of the central nervous system (CNS) in adults and children over 2 years of age to visualize lesions with abnormal blood brain barrier or abnormal vascularity of the brain, spine, and associated tissues. /Included in US product labeling/
NIH; DailyMed. Current Medication Information for MultiHance (Gadobenate Dimeglumine) Injection, Solution (Revised: July 2013). Available from, as of October 27, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=59077a3e-5f03-4342-ae24-856267545631
MultiHance is indicated for use in magnetic resonance angiography (MRA) to evaluate adults with known or suspected renal or aorto-ilio-femoral occlusive vascular disease. /Included in US product label/
NIH; DailyMed. Current Medication Information for MultiHance (Gadobenate Dimeglumine) Injection, Solution (Revised: July 2013). Available from, as of October 27, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=59077a3e-5f03-4342-ae24-856267545631
A study was conducted to compare gadobenate dimeglumine (Multihance) with other commercially available MRI contrast agents for the detection of intracranial metastases ... A retrospective assessment was performed on MR images from 22 patients enrolled in a prior phase II clinical trial of gadobenate dimeglumine. Each patient underwent two examinations: a first examination with one of three "comparator" agents (gadopentetate dimeglumine, gadodiamide, and gadoterate meglumine) at a dosage of either 0.1 or 0.2 mmol/kg, and then a similar examination with gadobenate dimeglumine at equal dosage. All images were evaluated randomly for lesion number and location in unpaired and then paired fashion by two independent, masked neuroradiologists. A third assessor performed quantitative assessments on the available complete sets of digitally recorded images (10 cases) ... The findings for the comparator agents were pooled. Sensitivity for lesion detection with gadobenate dimeglumine (93%-100%) was markedly superior to that of comparator-enhanced examinations (65%-73%). The increase of lesion-to-brain contrast of the main lesion was consistently greater with gadobenate dimeglumine than with comparator agents relative to unenhanced contrast (+43% vs. +27%) ... Gadobenate dimeglumine proved to be a more efficacious agent than comparator contrast agents for the detection of intracranial metastatic lesions: superior efficacy was noted by both reviewers for total lesion count as well as for sensitivity and positive predictive value for lesion detection. The higher relaxivity of gadobenate dimeglumine might explain the superior sensitivity of gadobenate dimeglumine-enhanced MRI for the detection of central nervous system metastases.
PMID:11224754 Colosimo C et al; Invest Radiol 36 (2): 72-81 (2001)
For more Therapeutic Uses (Complete) data for GADOBENATE DIMEGLUMINE (9 total), please visit the HSDB record page.
/BOXED WARNING/ WARNING: NEPHROGENIC SYSTEMIC FIBROSIS. Gadolinium-based contrast agents (GBCAs) increase the risk for nephrogenic systemic fibrosis (NSF) among patients with impaired elimination of the drugs. Avoid use of GBCAs in these patients unless the diagnostic information is essential and not available with non-contrasted MRI or other modalities. NSF may result in fatal or debilitating systemic fibrosis affecting the skin, muscle and internal organs. The risk for NSF appears highest among patients with: chronic, severe kidney disease (GFR <30 mL/min/1.73 sq m), or acute kidney injury. Screen patients for acute kidney injury and other conditions that may reduce renal function. For patients at risk for chronically reduced renal function (e.g. age > 60 years, hypertension or diabetes), estimate the glomerular filtration rate (GFR) through laboratory testing. For patients at highest risk for NSF, do not exceed the recommended MultiHance dose and allow a sufficient period of time for elimination of the drug from the body prior to re-administration.
NIH; DailyMed. Current Medication Information for MultiHance (Gadobenate Dimeglumine) Injection, Solution (Revised: July 2013). Available from, as of October 27, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=59077a3e-5f03-4342-ae24-856267545631
Gadolinium-based contrast agents (GBCAs) increase the risk for nephrogenic systemic fibrosis (NSF) among patients with impaired elimination of the drugs. Avoid use of GBCAs among these patients unless the diagnostic information is essential and not available with non-contrast enhanced MRI or other modalities. The GBCA-associated NSF risk appears highest for patients with chronic, severe kidney disease (GFR <30 mL/min/1.73 sq m) as well as patients with acute kidney injury. The risk appears lower for patients with chronic, moderate kidney disease (GFR 30-59 mL/min/1.73 sq m) and little, if any, for patients with chronic, mild kidney disease (GFR 60-89 mL/min/1.73 sq m). NSF may result in fatal or debilitating fibrosis affecting the skin, muscle and internal organs. ... Screen patients for acute kidney injury and other conditions that may reduce renal function. Features of acute kidney injury consist of rapid (over hours to days) and usually reversible decrease in kidney function, commonly in the setting of surgery, severe infection, injury or drug-induced kidney toxicity. Serum creatinine levels and estimated GFR may not reliably assess renal function in the setting of acute kidney injury. For patients at risk for chronically reduced renal function (e.g., age > 60 years, diabetes mellitus or chronic hypertension), estimate the GFR through laboratory testing. Among the factors that may increase the risk for NSF are repeated or higher than recommended doses of a GBCA and the degree of renal impairment at the time of exposure. Record the specific GBCA and the dose administered to a patient. For patients at highest risk for NSF, do not exceed the recommended MultiHance dose and allow a sufficient period of time for elimination of the drug prior to re-administration. For patients receiving hemodialysis, physicians may consider the prompt initiation of hemodialysis following the administration of a GBCA in order to enhance the contrast agent's elimination. The usefulness of hemodialysis in the prevention of NSF is unknown
NIH; DailyMed. Current Medication Information for MultiHance (Gadobenate Dimeglumine) Injection, Solution (Revised: July 2013). Available from, as of October 27, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=59077a3e-5f03-4342-ae24-856267545631
Anaphylactic and anaphylactoid reactions have been reported, involving cardiovascular, respiratory, and/or cutaneous manifestations. Some patients experienced circulatory collapse and died. In most cases, initial symptoms occurred within minutes of MultiHance administration and resolved with prompt emergency treatment. Prior to MultiHance administration, ensure the availability of personnel trained and medications to treat hypersensitivity reactions. If such a reaction occurs stop MultiHance and immediately begin appropriate therapy. Additionally, consider the risk for hypersensitivity reactions, especially in patients with a history of hypersensitivity reactions or a history of asthma or other allergic disorders. Observe patients for signs and symptoms of a hypersensitivity reaction during and for up to 2 hours after MultiHance administration.
NIH; DailyMed. Current Medication Information for MultiHance (Gadobenate Dimeglumine) Injection, Solution (Revised: July 2013). Available from, as of October 27, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=59077a3e-5f03-4342-ae24-856267545631
FDA Pregnancy Risk Category: C /RISK CANNOT BE RULED OUT. Adequate, well controlled human studies are lacking, and animal studies have shown risk to the fetus or are lacking as well. There is a chance of fetal harm if the drug is given during pregnancy; but the potential benefits may outweigh the potential risk./
NIH; DailyMed. Current Medication Information for MultiHance (Gadobenate Dimeglumine) Injection, Solution (Revised: July 2013). Available from, as of October 27, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=59077a3e-5f03-4342-ae24-856267545631
For more Drug Warnings (Complete) data for GADOBENATE DIMEGLUMINE (15 total), please visit the HSDB record page.
Contrast Media
Substances used to allow enhanced visualization of tissues. (See all compounds classified as Contrast Media.)
Gadobenate ion has a rapid distribution half-life (reported as mean + or - SD) of 0.084 + or - 0.012 to 0.605 + or - 0.072 hours. Volume of distribution of the central compartment ranged from 0.074:: 0.017 to 0.158 :: 0.038 L/kg, and estimates of volume of distribution by area ranged from 0.170+ or - 0.016 to 0.282+ or - 0.079 L/kg. These latter estimates are approximately equivalent to the average volume of extracellular body water in man. In vitro studies showed no appreciable binding of gadobenate ion to human serum proteins. /Gadobenate ion/
NIH; DailyMed. Current Medication Information for MultiHance (Gadobenate Dimeglumine) Injection, Solution (Revised: July 2013). Available from, as of October 27, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=59077a3e-5f03-4342-ae24-856267545631
... The pharmacokinetics were evaluated in rats, rabbits, dogs and monkeys after iv injections of non-labelled gadobenate dimeglumine and, for biodistribution studies, (153)-Gd-labelled gadobenate dimeglumine. Assays were performed by high performance liquid chromatography, X-ray fluorescence and gamma spectrometry. The binding of gadobenate ion to animal and human serum albumin was studied by equilibrium dialysis. ... After iv injection gadobenate dimeglumine distributes into plasma and extracellular fluid as well as into the intrahepatocytic space. Gadobenate ion is cleared from plasma by renal and biliary excretion. It does not accumulate in specific tissues, except temporarily in tissues related to its elimination. Gadobenate ion is not metabolized. Its binding to plasma proteins is too weak to be detected by equilibrium dialysis. ...
PMID:10608414 Lorusso V et al; J Comput Assist Tomogr 23 Suppl 1:S181-94 (1999)
Gadobenate ion is eliminated predominately via the kidneys, with 78% to 96% of an administered dose recovered in the urine. Total plasma clearance and renal clearance estimates of gadobenate ion were similar, ranging from 0.093 + or - 0.010 to 0.133 + o r- 0.270 L/hr/kg and 0.082+ or - 0.007 to 0.104 + or - 0.039 L/hr/kg, respectively. The clearance is similar to that of substances that are subject to glomerular filtration. The mean elimination half-life ranged from 1.17+ or - 0.26 to 2.02 + or - 0.60 hours. A small percentage ofthe administered dose (0.6% to 4%) is eliminated via the biliary route and recovered in feces.
NIH; DailyMed. Current Medication Information for MultiHance (Gadobenate Dimeglumine) Injection, Solution (Revised: July 2013). Available from, as of October 27, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=59077a3e-5f03-4342-ae24-856267545631
It is not known to what extent gadobenate dimeglumine is excreted in human milk. It is known from rat experiments that less than 0.5% of the administered dose is transferred via milk from mother to neonates.
NIH; DailyMed. Current Medication Information for MultiHance (Gadobenate Dimeglumine) Injection, Solution (Revised: July 2013). Available from, as of October 27, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=59077a3e-5f03-4342-ae24-856267545631
For more Absorption, Distribution and Excretion (Complete) data for GADOBENATE DIMEGLUMINE (7 total), please visit the HSDB record page.
There was no detectable biotransformation of gadobenate ion. Dissociation of gadobenate ion in vivo has been shown to be minimal, with less than 1% of the free chelating agent being recovered alone in feces.
NIH; DailyMed. Current Medication Information for MultiHance (Gadobenate Dimeglumine) Injection, Solution (Revised: July 2013). Available from, as of October 27, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=59077a3e-5f03-4342-ae24-856267545631
Gadobenate ion is eliminated predominately via the kidneys, with 78% to 96% of an administered dose recovered in the urine. ... The mean elimination half-life ranged from 1.17+ or - 0.26 to 2.02 + or - 0.60 hours. ...
NIH; DailyMed. Current Medication Information for MultiHance (Gadobenate Dimeglumine) Injection, Solution (Revised: July 2013). Available from, as of October 27, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=59077a3e-5f03-4342-ae24-856267545631
... Fifteen children scheduled to undergo contrast-enhanced MRI for suspected disease of the central nervous system received a single intravenous injection of 0.1 mmol/kg gadobenate dimeglumine. ... 1.2 hr for terminal elimination half-life were determined across all age groups combined.
PMID:24807269 Pirovano G et al; J Magn Reson Imaging. 2014 May 8. doi: 10.1002/jmri.24653. (Epub ahead of print)
A single intravenous dose of 0.2 mmol/kg of Multihance was administered to 11 subjects (5 males and 6 females) with end-stage renal disease requiring hemodialysis ... . The mean elimination half-life on dialysis was 1.21 + or - 0.29 hours as compared with 42.4 + or - 24.4 hours when off dialysis.
NIH; DailyMed. Current Medication Information for MultiHance (Gadobenate Dimeglumine) Injection, Solution (Revised: July 2013). Available from, as of October 27, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=59077a3e-5f03-4342-ae24-856267545631
A single intravenous dose of 0.2 mmol/kg of Multihance was administered to 20 subjects with impaired renal function (6 men and 3 women with moderate renal impairment (urine creatinine clearance > 30 to < 60 mL/min) and 5 men and 6 women with severe renal impairment (urine creatinine clearance > 10 to < 30 mL/min)). Mean estimates of the elimination half-life were 6.1 + or - 3.0 and 9.5 + or - 3.1 hours for the moderate and severe renal impairment groups, respectively as compared with 1.0 to 2.0 hours in healthy volunteers.
NIH; DailyMed. Current Medication Information for MultiHance (Gadobenate Dimeglumine) Injection, Solution (Revised: July 2013). Available from, as of October 27, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=59077a3e-5f03-4342-ae24-856267545631
Gadobenate ion has a rapid distribution half-life (reported as mean + or - SD) of 0.084 + or - 0.012 to 0.605 + or - 0.072 hours.
NIH; DailyMed. Current Medication Information for MultiHance (Gadobenate Dimeglumine) Injection, Solution (Revised: July 2013). Available from, as of October 27, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=59077a3e-5f03-4342-ae24-856267545631
Global Sales Information

ABOUT THIS PAGE
91
PharmaCompass offers a list of Gadobenic Acid API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Gadobenic Acid manufacturer or Gadobenic Acid supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Gadobenic Acid manufacturer or Gadobenic Acid supplier.
PharmaCompass also assists you with knowing the Gadobenic Acid API Price utilized in the formulation of products. Gadobenic Acid API Price is not always fixed or binding as the Gadobenic Acid Price is obtained through a variety of data sources. The Gadobenic Acid Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Gadobenic Acid manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Gadobenic Acid, including repackagers and relabelers. The FDA regulates Gadobenic Acid manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Gadobenic Acid API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Gadobenic Acid manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Gadobenic Acid supplier is an individual or a company that provides Gadobenic Acid active pharmaceutical ingredient (API) or Gadobenic Acid finished formulations upon request. The Gadobenic Acid suppliers may include Gadobenic Acid API manufacturers, exporters, distributors and traders.
click here to find a list of Gadobenic Acid suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Gadobenic Acid as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Gadobenic Acid API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Gadobenic Acid as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Gadobenic Acid and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Gadobenic Acid NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Gadobenic Acid suppliers with NDC on PharmaCompass.
Gadobenic Acid Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Gadobenic Acid GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Gadobenic Acid GMP manufacturer or Gadobenic Acid GMP API supplier for your needs.
A Gadobenic Acid CoA (Certificate of Analysis) is a formal document that attests to Gadobenic Acid's compliance with Gadobenic Acid specifications and serves as a tool for batch-level quality control.
Gadobenic Acid CoA mostly includes findings from lab analyses of a specific batch. For each Gadobenic Acid CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Gadobenic Acid may be tested according to a variety of international standards, such as European Pharmacopoeia (Gadobenic Acid EP), Gadobenic Acid JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Gadobenic Acid USP).
