
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDF
0
Australia

0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
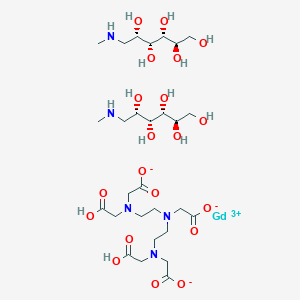

1. Diethylenetriaminepenta-acetic Acid, Gadolinium
2. Dimeglumine, Gadolinium Dtpa
3. Dimeglumine, Gadopentetate
4. Dtpa, Gadolinium
5. Gadolinium Diethylenetriaminepenta Acetic Acid
6. Gadolinium Diethylenetriaminepenta-acetic Acid
7. Gadolinium Dtpa
8. Gadolinium Dtpa Dimeglumine
9. Gadolinium Dtpa Dimeglumine Salt
10. Gadolinium Dtpa Disodium Salt
11. Gadopentetate Dimeglumine
12. Gadopentetic Acid
13. Gd Dtpa
14. Gd-dtpa
15. Magnevist Enteral
16. Magnograf
17. Magnograf Enteral
1. Gadopentetic Acid Dimeglumine Salt
2. 2-[bis[2-[carboxylatomethyl(carboxymethyl)amino]ethyl]amino]acetate;gadolinium(3+);(2r,3r,4r,5s)-6-(methylamino)hexane-1,2,3,4,5-pentol
3. Gadopentetic Acid Dimeglumine
4. Chebi:31797
5. Sh L 451a
6. Sh-l-451a
7. Dtxsid70235367
8. Akos015896299
9. Bay-86-4882
10. D01707
11. Q413793
12. Gadolinium (bis{2-[(carboxylatomethyl)(carboxymethyl)amino]ethyl}amino)acetate--1-deoxy-1-(methylamino)-d-glucitol (1:2)
| Molecular Weight | 938.0 g/mol |
|---|---|
| Molecular Formula | C28H54GdN5O20 |
| Hydrogen Bond Donor Count | 14 |
| Hydrogen Bond Acceptor Count | 25 |
| Rotatable Bond Count | 25 |
| Exact Mass | 938.26033 g/mol |
| Monoisotopic Mass | 938.26033 g/mol |
| Topological Polar Surface Area | 431 Ų |
| Heavy Atom Count | 54 |
| Formal Charge | 0 |
| Complexity | 631 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 8 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 4 |
| 1 of 2 | |
|---|---|
| Drug Name | Magnevist |
| PubMed Health | Gadopentetate (Injection) |
| Drug Classes | Radiological Non-Ionic Contrast Media |
| Drug Label | Magnevist (brand of gadopentetate dimeglumine) injection is the N-methylglucamine salt of the gadolinium complex of diethylenetriamine pentaacetic acid, and is an injectable contrast medium for magnetic resonance imaging (MRI). Magnevist Injection... |
| Active Ingredient | Gadopentetate dimeglumine |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 469.01mg/ml |
| Market Status | Prescription |
| Company | Bayer Hlthcare |
| 2 of 2 | |
|---|---|
| Drug Name | Magnevist |
| PubMed Health | Gadopentetate (Injection) |
| Drug Classes | Radiological Non-Ionic Contrast Media |
| Drug Label | Magnevist (brand of gadopentetate dimeglumine) injection is the N-methylglucamine salt of the gadolinium complex of diethylenetriamine pentaacetic acid, and is an injectable contrast medium for magnetic resonance imaging (MRI). Magnevist Injection... |
| Active Ingredient | Gadopentetate dimeglumine |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 469.01mg/ml |
| Market Status | Prescription |
| Company | Bayer Hlthcare |
Contrast Media
Substances used to allow enhanced visualization of tissues. (See all compounds classified as Contrast Media.)
DRUG PRODUCT COMPOSITIONS

Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?