
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
API
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
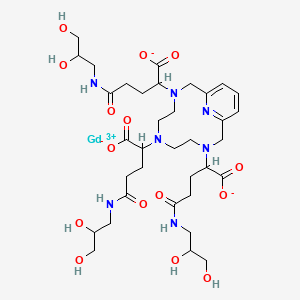

1. 3,6,9,15-tetraazabicyclo(9.3.1)pentadeca-1(15),11,13-triene-3,6,9-triacetic Acid, Alpha, Alpha', Alpha''-tris(3-((2,3-dihydroxypropyl)amino)-3-oxopropyl)-, Gadolinium Salt (1:1)
2. Gadolinium 2,2',2''-(3,6,9,15-tetraazabicyclo(9.3.1)pentadeca-1(15),11,13-triene-3,6,9-triyl)tris(5-((2,3-dihydroxypropyl)amino)-5-oxopentanoate)
1. Unii-s276568koy
2. Gadopiclenol [usan]
3. S276568koy
4. Elucirem
5. P03277
6. Who 10744
7. 933983-75-6
8. P-03277
9. (alpha3,alpha6,alpha9-tris(3-((2,3-dihydroxypropyl)amino)-3-oxopropyl)-3,6,9,15-tetraazabicyclo(9.3.1)pentadeca-1(15),11,13-triene-3,6,9-triacetato(3-)-kappan3,kappan6,kappan9,kappan15,kappao3,kappao6,kappao9)gadolinium
10. 2-[3,9-bis[1-carboxylato-4-(2,3-dihydroxypropylamino)-4-oxobutyl]-3,6,9,15-tetrazabicyclo[9.3.1]pentadeca-1(15),11,13-trien-6-yl]-5-(2,3-dihydroxypropylamino)-5-oxopentanoate;gadolinium(3+)
11. Gadopiclenolum
12. Vueway
13. Gadopiclenol [inn]
14. Schembl21682611
15. Gbca P03277
16. P 03277
17. Gadolinium-based Magnetic Resonance Contrast Agent P03277
18. (.alpha.3,.alpha.6,.alpha.9-tris(3-((2,3-dihydroxypropyl)amino)-3-oxopropyl)-3,6,9,15-tetraazabicyclo(9.3.1)pentadeca-1(15),11,13-triene-3,6,9-triacetato(3-)-.kappa.n3,.kappa.n6,.kappa.n9,.kappa.n15,.kappa.o3,.kappa.o6,.kappa.o9)gadolinium
19. Gadolinium, (.alpha.3, .alpha.6, .alpha.9-tris(3-((2-hydroxy-1-(hydroxymethyl)ethyl)amino)-3-oxopropyl)-3,6,9,15-tetraazabicyclo(9.3.1)pentadeca-1(15),11,13-triene-3,6,9-triacetato(3-)-.kappa.n3, .kappa.n6, .kappa.n9, .kappa.n15, .kappa.o3, .kappa.o6, .kappa.o9)
20. Gadolinium, (alpha3, Alpha6, Alpha9-tris(3-((2-hydroxy-1-(hydroxymethyl)ethyl)amino)-3-oxopropyl)-3,6,9,15-tetraazabicyclo(9.3.1)pentadeca-1(15),11,13-triene-3,6,9-triacetato(3-)-kappan3, Kappan6, Kappan9, Kappan15, Kappao3, Kappao6, Kappao9)
| Molecular Weight | 970.1 g/mol |
|---|---|
| Molecular Formula | C35H54GdN7O15 |
| Hydrogen Bond Donor Count | 9 |
| Hydrogen Bond Acceptor Count | 19 |
| Rotatable Bond Count | 21 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 352 |
| Heavy Atom Count | 58 |
| Formal Charge | 0 |
| Complexity | 1200 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 6 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Gadopiclenol is indicated in adult and pediatric patients aged 2 years and older for use with magnetic resonance imaging (MRI) to detect and visualize lesions with abnormal vascularity in the central nervous system (brain, spine, and associated tissues) and the body (head and neck, thorax, abdomen, pelvis, and musculoskeletal system).
Detection and visualization of disorders or lesions with suspected abnormal vascularity in various body regions for diagnostic purposes
Detection and visualisation of areas with disruption of the blood brain barrier and/or abnormal vascularity for diagnostic purposes
V - Various
V08 - Contrast media
V08C - Magnetic resonance imaging contrast media
V08CA - Paramagnetic contrast media
V08CA12 - Gadopiclenol
Absorption
At a dose range between 0.025 mmol/kg and 0.3 mmol/kg (0.5 times to 6 times the recommended dosage), the Cmax and AUCinf of gadopiclenol increases in a dose-proportional manner. At the recommended dose, gadopiclenol has a Cmax of 525 g/mL and an AUCinf of 569 gh/mL. A study that evaluated the pharmacokinetic parameters of gadopiclenol in healthy subjects and patients with brain lesions did not detect significant differences between the two groups.
Route of Elimination
Gadopiclenol is mainly excreted in urine by glomerular filtration. Within 48 hours after administration, approximately 98% of the gadopiclenol dose was recovered in urine.
Volume of Distribution
At steady state, the mean volume of distribution of gadopiclenol is 13 L.
Clearance
Gadopiclenol has a total body clearance of 100 mL/min and a renal clearance of 81 mL/min.
Gadopiclenol is not metabolized and is eliminated unchanged.
Gadopiclenol has a mean elimination half-life of is 1.5 hours.
Gadopiclenol is a macrocyclic non-ionic complex of gadolinium and a paramagnetic molecule that develops a magnetic moment when placed in a magnetic field. The magnetic moment alters the relaxation rates of water protons in its vicinity in the body. Its use in magnetic resonance imaging (MRI) allows to selectively increase contrast in tissues where gadopiclenol accumulates.
Global Sales Information

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
