
Synopsis
Synopsis
0
CEP/COS
0
KDMF
0
VMF
0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
API
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
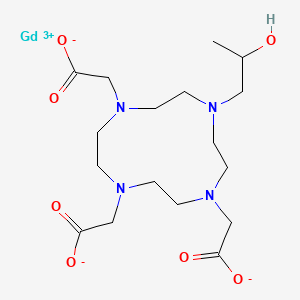

1. Gadolinium 1,4,7-tris(carboxymethyl)-10-(2'-hydroxypropyl)-1,4,7,10-tetraazacyclododecane
2. Gadolinium 1,4,7-triscarboxymethyl-1,4,7,10-tetraazacyclododecane
3. Gadolinium Hp-do3a
4. Gd(do3a)
5. Gd-hp-d03a
6. Gd-hp-do3a
7. Gd-hpdo3a
8. Gd-hydroxypropyl-d03a
9. Gdhpdo3a
10. Prohance
11. Sq 32,692
12. Sq 32692
13. Sq-32692
1. Prohance
2. 120066-54-8
3. Sq-32692
4. Dsstox_cid_28588
5. Dsstox_rid_82859
6. Dsstox_gsid_48662
7. Gd-hpdo3a
8. Gd-hp-do3a
9. Cas-120066-54-8
10. 0199mv609f
11. Nsc-760055
12. Ncgc00182068-02
13. Ncgc00182068-03
14. Sq 32,692
15. Prohance (tn)
16. Gadoteridol [mi]
17. Gadolinium 2,2',2''-[10-(2-hydroxypropyl)-1,4,7,10-tetraazacyclododecane-1,4,7-triyl]triacetate
18. Gadoteridol [inn]
19. Gadoteridol [jan]
20. Gd(hp-do3a)
21. Gadoteridol [hsdb]
22. Gadoteridol [usan]
23. Gadoteridol [vandf]
24. Gadoteridol [mart.]
25. Gadoteridol [usp-rs]
26. Gadoteridol [who-dd]
27. Gadoteridol (jan/usp/inn)
28. Schembl1650466
29. Dtxsid2048662
30. Gadoteridol [orange Book]
31. Gadoteridol [usp Impurity]
32. Hms3264d11
33. Moli001032
34. Gadoteridol [usp Monograph]
35. Bcp13402
36. Tox21_113025
37. Tox21_113128
38. Tox21_113025_1
39. Ccg-213206
40. Db00597
41. Ncgc00182068-04
42. 2-[4,7-bis(carboxylatomethyl)-10-(2-hydroxypropyl)-1,4,7,10-tetrazacyclododec-1-yl]acetate;gadolinium(3+)
43. D01137
44. Q5516429
45. (+/-)-(10-(2-hydroxypropyl)-1,4,7,10-tetraazacyclododecane-1,4,7-triacetato(3-))gadolinium.
46. Gadolinium, (10-(2-hydroxypropyl)-1,4,7,10-tetraazacyclododecane-1,4,7-triacetato(3-)-n1,n4,n7,n10,o1,o4,o7,o10)-
| Molecular Weight | 558.7 g/mol |
|---|---|
| Molecular Formula | C17H29GdN4O7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 5 |
| Exact Mass | 559.12774 g/mol |
| Monoisotopic Mass | 559.12774 g/mol |
| Topological Polar Surface Area | 154 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 471 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 4 | |
|---|---|
| Drug Name | Prohance |
| PubMed Health | Gadoteridol (Injection) |
| Drug Classes | Radiological Non-Ionic Contrast Media |
| Drug Label | ProHance (Gadoteridol) Injection is a nonionic contrast medium for magnetic resonance imaging (MRI), available as a 0.5M sterile clear colorless to slightly yellow aqueous solution in vials and syringes for intravenous injection.Gadoteridol is the ga... |
| Active Ingredient | Gadoteridol |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 279.3mg/ml |
| Market Status | Prescription |
| Company | Bracco |
| 2 of 4 | |
|---|---|
| Drug Name | Prohance multipack |
| Active Ingredient | Gadoteridol |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 279.3mg/ml |
| Market Status | Prescription |
| Company | Bracco |
| 3 of 4 | |
|---|---|
| Drug Name | Prohance |
| PubMed Health | Gadoteridol (Injection) |
| Drug Classes | Radiological Non-Ionic Contrast Media |
| Drug Label | ProHance (Gadoteridol) Injection is a nonionic contrast medium for magnetic resonance imaging (MRI), available as a 0.5M sterile clear colorless to slightly yellow aqueous solution in vials and syringes for intravenous injection.Gadoteridol is the ga... |
| Active Ingredient | Gadoteridol |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 279.3mg/ml |
| Market Status | Prescription |
| Company | Bracco |
| 4 of 4 | |
|---|---|
| Drug Name | Prohance multipack |
| Active Ingredient | Gadoteridol |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 279.3mg/ml |
| Market Status | Prescription |
| Company | Bracco |
Contrast Media
National Library of Medicine's Medical Subject Headings. Gadoteridol. Online file (MeSH, 2014). Available from, as of October 23, 2014: https://www.nlm.nih.gov/mesh/2014/mesh_browser/MBrowser.html
ProHance (Gadoteridol) Injection is indicated for use in MRI in adults and children over 2 years of age to visualize lesions with abnormal vascularity in the brain (intracranial lesions), spine and associated tissues. /Included in US product label/
NIH; DailyMed. Current Medication Information for ProHance (Gadoteridol) Injection, Solution (Revised: September 2011). Available from, as of October 29, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=778aee03-7d4c-481a-be8a-a75db0702f5a
ProHance is indicated for use in MRI in adults to visualize lesions in the head and neck. /Included in US product label/
NIH; DailyMed. Current Medication Information for ProHance (Gadoteridol) Injection, Solution (Revised: September 2011). Available from, as of October 29, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=778aee03-7d4c-481a-be8a-a75db0702f5a
/BOXED WARNING/ WARNING: NEPHROGENIC SYSTEMIC FIBROSIS. Gadolinium-based contrast agents (GBCAs) increase the risk for nephrogenic systemic fibrosis (NSF) among patients with impaired elimination of the drugs. Avoid use of GBCAs in these patients unless the diagnostic information is essential and not available with non-contrasted MRI or other modalities. NSF may result in fatal or debilitating systemic fibrosis affecting the skin, muscle and internal organs. The risk for NSF appears highest among patients with: chronic, severe kidney disease (GFR <30 mL/min/1.73 sq m), or acute kidney injury. Screen patients for acute kidney injury and other conditions that may reduce renal function. For patients at risk for chronically reduced renal function (e.g. age > 60 years, hypertension or diabetes), estimate the glomerular filtration rate (GFR) through laboratory testing. For patients at highest risk for NSF, do not exceed the recommended ProHance dose and allow a sufficient period of time for elimination of the drug from the body prior to re-administration.
NIH; DailyMed. Current Medication Information for ProHance (Gadoteridol) Injection, Solution (Revised: September 2011). Available from, as of October 29, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=778aee03-7d4c-481a-be8a-a75db0702f5a
Gadolinium-based contrast agents (GBCAs) increase the risk for nephrogenic systemic fibrosis (NSF) among patients with impaired elimination of the drugs. Avoid use of GBCAs among these patients unless the diagnostic information is essential and not available with non-contrast enhanced MRI or other modalities. The GBCA-associated NSF risk appears highest for patients with chronic, severe kidney disease (GFR <30 mL/min/1.73 sq m) as well as patients with acute kidney injury. The risk appears lower for patients with chronic, moderate kidney disease (GFR 30-59 mL/min/1.73 sq m) and little, if any, for patients with chronic, mild kidney disease (GFR 60-89 mL/min/1.73 sq m). NSF may result in fatal or debilitating fibrosis affecting the skin, muscle and internal organs. ... Screen patients for acute kidney injury and other conditions that may reduce renal function. Features of acute kidney injury consist of rapid (over hours to days) and usually reversible decrease in kidney function, commonly in the setting of surgery, severe infection, injury or drug-induced kidney toxicity. Serum creatinine levels and estimated GFR may not reliably assess renal function in the setting of acute kidney injury. For patients at risk for chronically reduced renal function (e.g., age > 60 years, diabetes mellitus or chronic hypertension), estimate the GFR through laboratory testing. Among the factors that may increase the risk for NSF are repeated or higher than recommended doses of a GBCA and the degree of renal impairment at the time of exposure. Record the specific GBCA and the dose administered to a patient. For patients at highest risk for NSF, do not exceed the recommended ProHance dose and allow a sufficient period of time for elimination of the drug prior to re-administration. For patients receiving hemodialysis, physicians may consider the prompt initiation of hemodialysis following the administration of a GBCA in order to enhance the contrast agent's elimination. The usefulness of hemodialysis in the prevention of NSF is unknown
NIH; DailyMed. Current Medication Information for ProHance (Gadoteridol) Injection, Solution (Revised: September 2011). Available from, as of October 29, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=778aee03-7d4c-481a-be8a-a75db0702f5a
The possibility of a reaction, including serious, life threatening, or fatal, anaphylactic or cardiovascular reactions, or other idiosyncratic reactions, should always be considered, especially in those patients with a history of a known clinical hypersensitivity or a history of asthma or other allergic respiratory disorders.
NIH; DailyMed. Current Medication Information for ProHance (Gadoteridol) Injection, Solution (Revised: September 2011). Available from, as of October 29, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=778aee03-7d4c-481a-be8a-a75db0702f5a
FDA Pregnancy Risk Category: C /RISK CANNOT BE RULED OUT. Adequate, well controlled human studies are lacking, and animal studies have shown risk to the fetus or are lacking as well. There is a chance of fetal harm if the drug is given during pregnancy; but the potential benefits may outweigh the potential risk./
NIH; DailyMed. Current Medication Information for ProHance (Gadoteridol) Injection, Solution (Revised: September 2011). Available from, as of October 29, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=778aee03-7d4c-481a-be8a-a75db0702f5a
For more Drug Warnings (Complete) data for GADOTERIDOL (19 total), please visit the HSDB record page.
Gadoteridol is an MRI contrast agent used for contrast enhancement of the brain, spine and surrounding tissues resulting in improved visualization (compared with unenhanced MRI) of lesions with abnormal vascularity or those thought to cause a disruption of the normal blood brain barrier. Gadoteridol can also be used for whole body contrast enhanced MRI including the head, neck, liver, breast, musculoskeletal system and soft tissue pathologies.
FDA Label
Contrast Media
Substances used to allow enhanced visualization of tissues. (See all compounds classified as Contrast Media.)
V08CA04
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
V - Various
V08 - Contrast media
V08C - Magnetic resonance imaging contrast media
V08CA - Paramagnetic contrast media
V08CA04 - Gadoteridol
Route of Elimination
Gadoteridol is eliminated in the urine with 94.4 4.8% (mean SD) of the dose excreted within 24 hours post-injection.
Volume of Distribution
204 58 mL/kg
Clearance
1.50+/- 0.35 mL/ min/kg
renal cl=1.41 +/- 0.33 mL/ min/kg
... The physicochemical properties of gadoteridol, a macrocyclic nonionic gadolinium complex, were studied together with its pharmacokinetics and biodistribution in rats and dogs. ... Studies in rats were conducted after single iv injections at 0.1 or 0.35 mmol/kg using (153)Gd-labeled gadoteridol or with seven daily doses of 0.1 mmol/kg to examine the levels of residual gadolinium in organs. Nonradioactive biodistribution and excretion studies were performed in dogs following injection at 0.1 mmol/kg. ... After injection, the dose was rapidly cleared from rat blood and excreted such that more than 90% of the dose appeared in the urine within 4 hr of injection. At 7 and 14 days postinjection, only extremely low levels of gadolinium were observed in liver and bone; these levels were two to eight times lower than the levels reported after the injection of gadopentetate dimeglumine. ... Differences observed in the long-term retention of gadolinium between gadoteridol and gadopentetate dimeglumine were consistent with the reported greater in vivo resistance to transmetallation of gadolinium macrocycles compared with the linear gadolinium chelate molecules.
PMID:9419608 Eakins MN et al; Acad Radiol 2 (7): 584-91 (1995)
... To assess the safety and pharmacokinetics of gadoteridol injection (0.5 M) ... 18 healthy male volunteers ... were assigned to one of six dosing groups: 0.05, 0.1, 0.15, 0.2, 0.25, and 0.3 mmol/kg gadoteridol (0.5 M), in an ascending dose study. Physical examination, vital signs, electrocardiogram, clinical laboratory tests, and serum and urine samples were obtained at selected time points before and after administration of gadoteridol. ... No significant changes in vital signs, physical examination, clinical laboratory values, or electrocardiogram, that were believed ... to be related to the administration of the contrast agent, were observed. A single adverse event (transient hive) believed to be related to contrast agent administration was observed in one volunteer. Pharmacokinetic data show that the elimination half-life and the distribution half-life were independent of the dose used. The mean distribution half-life was 0.20 +/- 0.04 hours, the mean elimination half-life was 1.57 +/- 0.08 hours, and greater than 94% of the drug was excreted in the urine in 24 hours.
PMID:1506147 McLachlan SJ et al; Invest Radiol 27 Suppl 1:S12-5 (1992)
Gadoteridol is eliminated in the urine with 94.4 + or - 4.8% (mean + or - SD) of the dose excreted within 24 hours post-injection.
NIH; DailyMed. Current Medication Information for ProHance (Gadoteridol) Injection, Solution (Revised: September 2011). Available from, as of October 29, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=778aee03-7d4c-481a-be8a-a75db0702f5a
Gadoteridol does not cross the intact blood-brain barrier and, therefore, does not accumulate in normal brain or in lesions that have a normal blood-brain barrer, e.g., cysts, mature post-operative scars, etc. However, disruption of the blood-brain barrer or abnormal vascularity allows accumulation of gadoteridol in lesions such as neoplasms, abscesses, and subacute infarcts. The pharmacokinetics of ProHance in various lesions is not known.
NIH; DailyMed. Current Medication Information for ProHance (Gadoteridol) Injection, Solution (Revised: September 2011). Available from, as of October 29, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=778aee03-7d4c-481a-be8a-a75db0702f5a
For more Absorption, Distribution and Excretion (Complete) data for GADOTERIDOL (7 total), please visit the HSDB record page.
It is unkown if biotransformation or decomposition of gadoteridol occur in vivo.
NIH; DailyMed. Current Medication Information for ProHance (Gadoteridol) Injection, Solution (Revised: September 2011). Available from, as of October 29, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=778aee03-7d4c-481a-be8a-a75db0702f5a
Distribution 12 minutes (mean), elimination 100 minutes (mean).
The pharmacokinetics of intravenously administered gadoteridol in normal subjects conforms to a two-compartment open model with mean distribution and elimination half-lives (reported as mean + or - SD) of about 0.20 + or - 0.04 hours and 1.57 + or - 0.08 hours, respectively.
NIH; DailyMed. Current Medication Information for ProHance (Gadoteridol) Injection, Solution (Revised: September 2011). Available from, as of October 29, 2014: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=778aee03-7d4c-481a-be8a-a75db0702f5a
... 18 healthy male volunteers ... were assigned to one of six dosing groups: 0.05, 0.1, 0.15, 0.2, 0.25, and 0.3 mmol/kg gadoteridol (0.5 M), in an ascending dose study. ...Pharmacokinetic data show that the elimination half-life and the distribution half-life were independent of the dose used. The mean distribution half-life was 0.20 +/- 0.04 hours, the mean elimination half-life was 1.57 +/- 0.08 hours... .
PMID:1506147 McLachlan SJ et al; Invest Radiol 27 Suppl 1:S12-5 (1992)
Based on the behavior of protons when placed in a strong magnetic field, which is interpreted and transformed into images by magnetic resonance (MR) instruments. Paramagnetic agents have unpaired electrons that generate a magnetic field about 700 times larger than the proton's field, thus disturbing the proton's local magnetic field. When the local magnetic field around a proton is disturbed its relaxation process is altered. MR images are based on proton density and proton relaxation dynamics. MR instruments can record two different relaxation processes, the T1 (spin-lattice or longitudinal relaxation time) and T2 (spin-spin or transverse relaxation time). In MRI, visualization of normal and pathological brain tissue depends in part on variations in the radiofrequency signal intensity that occur with changes in proton density, alteration of the T1, and variation in T2. When placed in a magnetic field, gadoteridol shortens the T1 relaxation time in tissues where it accumulates. Gadoteridol does not cross the intact blood-brain barrier; therefore, it does not accumulate in normal brain tissue or in central nervous system (CNS) lesions that have not caused an abnormal blood-brain barrier (e.g., cysts, mature post-operative scars). Abnormal vascularity or disruption of the blood-brain barrier allows accumulation of gadoteridol in lesions such as neoplasms, abscesses, and subacute infarcts.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
39
PharmaCompass offers a list of Gadoteridol API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Gadoteridol manufacturer or Gadoteridol supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Gadoteridol manufacturer or Gadoteridol supplier.
PharmaCompass also assists you with knowing the Gadoteridol API Price utilized in the formulation of products. Gadoteridol API Price is not always fixed or binding as the Gadoteridol Price is obtained through a variety of data sources. The Gadoteridol Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Gadoteridol manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Gadoteridol, including repackagers and relabelers. The FDA regulates Gadoteridol manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Gadoteridol API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Gadoteridol manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Gadoteridol supplier is an individual or a company that provides Gadoteridol active pharmaceutical ingredient (API) or Gadoteridol finished formulations upon request. The Gadoteridol suppliers may include Gadoteridol API manufacturers, exporters, distributors and traders.
click here to find a list of Gadoteridol suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Gadoteridol DMF (Drug Master File) is a document detailing the whole manufacturing process of Gadoteridol active pharmaceutical ingredient (API) in detail. Different forms of Gadoteridol DMFs exist exist since differing nations have different regulations, such as Gadoteridol USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Gadoteridol DMF submitted to regulatory agencies in the US is known as a USDMF. Gadoteridol USDMF includes data on Gadoteridol's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Gadoteridol USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Gadoteridol suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Gadoteridol Drug Master File in Japan (Gadoteridol JDMF) empowers Gadoteridol API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Gadoteridol JDMF during the approval evaluation for pharmaceutical products. At the time of Gadoteridol JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Gadoteridol suppliers with JDMF on PharmaCompass.
A Gadoteridol written confirmation (Gadoteridol WC) is an official document issued by a regulatory agency to a Gadoteridol manufacturer, verifying that the manufacturing facility of a Gadoteridol active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Gadoteridol APIs or Gadoteridol finished pharmaceutical products to another nation, regulatory agencies frequently require a Gadoteridol WC (written confirmation) as part of the regulatory process.
click here to find a list of Gadoteridol suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Gadoteridol as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Gadoteridol API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Gadoteridol as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Gadoteridol and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Gadoteridol NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Gadoteridol suppliers with NDC on PharmaCompass.
Gadoteridol Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Gadoteridol GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Gadoteridol GMP manufacturer or Gadoteridol GMP API supplier for your needs.
A Gadoteridol CoA (Certificate of Analysis) is a formal document that attests to Gadoteridol's compliance with Gadoteridol specifications and serves as a tool for batch-level quality control.
Gadoteridol CoA mostly includes findings from lab analyses of a specific batch. For each Gadoteridol CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Gadoteridol may be tested according to a variety of international standards, such as European Pharmacopoeia (Gadoteridol EP), Gadoteridol JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Gadoteridol USP).
