

Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
VMF
0
FDF
0
Australia

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
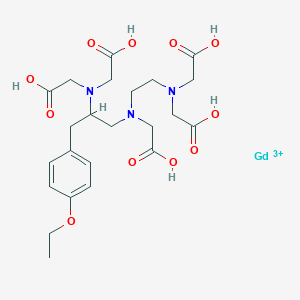

1. Disodium Gadoxetate
2. Eovist
3. Gadolinium (4s)-4-(4-ethoxybenzyl)-3,6,9-tris(carboxylatomethyl)-3,6,9-triazaundecanoic Acid Disodium Salt
4. Gadolinium Ethoxybenzyl Diethylenetriaminepentaacetic Acid
5. Gadolinium Ethoxybenzyl Dtpa
6. Gadoxetate Disodium
7. Gadoxetic Acid
8. Gadoxetic Acid Disodium
9. Gd-eob-dtpa
1. Eovist
2. Gadoxate
3. Gadoxate Disodium
4. Gadolinium Ethoxybenzyl-dtpa
5. Moli000482
6. Q1928394
7. 2-[[2-[bis(carboxymethyl)amino]-3-(4-ethoxyphenyl)propyl]-[2-[bis(carboxymethyl)amino]ethyl]amino]acetic Acid;gadolinium(3+)
8. Gadolinium-ethoxybenzyl-diethylenetriaminepentaacetic Acid, Disodium S-[4-(4-ethoxybenzyl)-3,6,9-tris[(carboxy-ko)methyl]-3,6,9-triazaundecandioato)(5-)-k3n3,n6,n9,k2o1,o11]gadolinite(2-)
| Molecular Weight | 684.8 g/mol |
|---|---|
| Molecular Formula | C23H33GdN3O11+3 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 14 |
| Rotatable Bond Count | 20 |
| Exact Mass | 685.13562 g/mol |
| Monoisotopic Mass | 685.13562 g/mol |
| Topological Polar Surface Area | 205 Ų |
| Heavy Atom Count | 38 |
| Formal Charge | 3 |
| Complexity | 739 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Eovist |
| PubMed Health | Gadoxetate (Injection) |
| Drug Classes | Diagnostic Agent |
| Drug Label | EOVIST (gadoxetate disodium) is a paramagnetic contrast agent for MRI. EOVIST is provided as a sterile, clear, colorless to pale yellow aqueous solution for intravenous injection.EOVIST contains the active pharmaceutical ingredient gadoxetate disodiu... |
| Active Ingredient | Gadoxetate disodium |
| Dosage Form | Solution |
| Route | Intravenous |
| Strength | 1.8143gm/10ml (181.43mg/ml); 2.72145gm/15ml (181.43mg/ml) |
| Market Status | Prescription |
| Company | Bayer Hlthcare |
| 2 of 2 | |
|---|---|
| Drug Name | Eovist |
| PubMed Health | Gadoxetate (Injection) |
| Drug Classes | Diagnostic Agent |
| Drug Label | EOVIST (gadoxetate disodium) is a paramagnetic contrast agent for MRI. EOVIST is provided as a sterile, clear, colorless to pale yellow aqueous solution for intravenous injection.EOVIST contains the active pharmaceutical ingredient gadoxetate disodiu... |
| Active Ingredient | Gadoxetate disodium |
| Dosage Form | Solution |
| Route | Intravenous |
| Strength | 1.8143gm/10ml (181.43mg/ml); 2.72145gm/15ml (181.43mg/ml) |
| Market Status | Prescription |
| Company | Bayer Hlthcare |
Contrast Media
Substances used to allow enhanced visualization of tissues. (See all compounds classified as Contrast Media.)
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
