

Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
Europe

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
Annual Reports
NA
Finished Drug Prices
NA
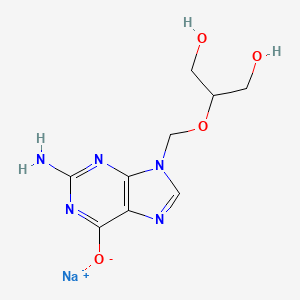

1. Biolf-62
2. Bw-759
3. Cytovene
4. Ganciclovir
5. Ganciclovir, Monosodium Salt
6. Gancyclovir
7. Rs-21592
1. 107910-75-8
2. Ganciclovir Sodium Salt
3. Cytovene Iv
4. Rs-21592 Sodium
5. Ganciclovir (sodium)
6. Ganciclovir Sodium [usan]
7. 84245-13-6
8. Sodium;2-amino-9-(1,3-dihydroxypropan-2-yloxymethyl)purin-6-olate
9. 107910-75-8 (sodium)
10. Ganciclovir Sodium (usan)
11. Natclovir
12. Cytovene-iv
13. 9-((2-hydroxy-1-(hydroxymethyl)ethoxy)methyl)guanine, Monosodium Salt
14. Rs 21592 Sodium
15. 02l083w284
16. Unii-02l083w284
17. Cytovene Iv Sodium
18. Ganciclovir Sodium (unspecified Mf)
19. Cytovene Iv (tn)
20. Ganciclovir (as Sodium)
21. 6h-purin-6-one, 2-amino-1,9-dihydro-9-((2-hydroxy-1-(hydroxymethyl)ethoxy)methyl)-, Monosodium Salt
22. Schembl149822
23. Chembl1200850
24. Dtxsid20883175
25. Hy-13637a
26. Mfcd00873979
27. Akos015896059
28. Bs-1004
29. Ccg-267214
30. Sodium 2-amino-1,9-dihydro-9-((2-hydroxy-1-(hydroxymethyl)ethoxy)methyl)-6h-purin-6-one
31. Db-059667
32. Cs-0013616
33. Ft-0656038
34. D04301
35. J-002034
36. Q27231533
37. Sodium 2-amino-9-((1,3-dihydroxypropan-2-yloxy)methyl)-9h-purin-6-olate
38. 2-amino-1,9-dihydro-9-[[2-hydroxy-1-(hydroxymethyl)ethoxy]methyl]-6h-purin-6-one Sodium Salt
39. 6h-purin-6-one, 1,9-dihydro-2-amino-9-((2-hydroxy-1-(hydroxymethyl)ethoxy)methyl)-, Monosodium Salt
40. 6h-purin-6-one, 2-amino-1,9-dihydro-9-((2-hydroxy-1-(hydroxymethyl)ethoxy)methyl)-, Sodium Salt
| Molecular Weight | 277.21 g/mol |
|---|---|
| Molecular Formula | C9H12N5NaO4 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 5 |
| Exact Mass | 277.07869816 g/mol |
| Monoisotopic Mass | 277.07869816 g/mol |
| Topological Polar Surface Area | 142 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 273 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 3 | |
|---|---|
| Drug Name | GANCICLOVIR SODIUM |
| Active Ingredient | GANCICLOVIR SODIUM |
| Company | PHARMASCIENCE INC (Application Number: A207645) |
| 2 of 3 | |
|---|---|
| Drug Name | GANCICLOVIR |
| Active Ingredient | GANCICLOVIR SODIUM |
| Company | FRESENIUS KABI USA (Application Number: A090658); LUITPOLD PHARMS INC (Application Number: A202624); MYLAN LABS LTD (Application Number: A204560); PAR STERILE PRODUCTS (Application Number: A204950) |
| 3 of 3 | |
|---|---|
| Drug Name | CYTOVENE |
| Active Ingredient | GANCICLOVIR SODIUM |
| Company | ROCHE PALO (Application Number: N019661) |
Antiviral Agents
Agents used in the prophylaxis or therapy of VIRUS DISEASES. Some of the ways they may act include preventing viral replication by inhibiting viral DNA polymerase; binding to specific cell-surface receptors and inhibiting viral penetration or uncoating; inhibiting viral protein synthesis; or blocking late stages of virus assembly. (See all compounds classified as Antiviral Agents.)

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ANALYTICAL

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
