
Synopsis
Synopsis
0
KDMF
0
VMF
0
FDF
0
Australia

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
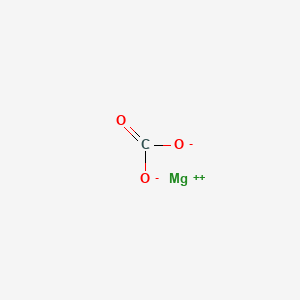

1. Anhydrous Magnesium Carbonate
2. C.i. 77713
3. Carbonic Acid, Magnesium Salt (1:1), Hydrate
4. Ci 77713
5. E-504
6. Magnesite
7. Magnesite (mg(co3))
8. Magnesium Carbonate (1:1) Hydrate
9. Magnesium Carbonate Anhydrous
10. Mgco3.3h2o
11. Nesquehonite
1. 546-93-0
2. Magnesite
3. Carbonic Acid, Magnesium Salt
4. 13717-00-5
5. Carbonic Acid, Magnesium Salt (1:1)
6. Magnesium Carbonate Anhydrous
7. Magnesite Dust
8. Magnesite (mg(co3))
9. Magnesium;carbonate
10. Hydromagnesite
11. Magmaster
12. Magnesium Carbonate (1:1)
13. 7757-69-9
14. Carbonate Magnesium
15. C.i. 77713
16. Stan-mag Magnesium Carbonate
17. Magnesium Carbonate,light
18. Magnesium Carbonate (mgco3)
19. Dci Light Magnesium Carbonate
20. Ins No.504(i)
21. Anhydrous Magnesium Carbonate
22. Ins-504(i)
23. Magnesium Carbonate Gold Star
24. Magnesium(ii) Carbonate (1:1)
25. Chebi:31793
26. E-504(i)
27. Mfcd00064632
28. Nsc-83511
29. 0ihc698356
30. Giobertite
31. Kimboshi
32. Apolda
33. Destab
34. Magfy
35. Magnesium Carbonate, Hydrated
36. Caswell No. 530
37. Gp 20 (carbonate)
38. Ma 70 (carbonate)
39. Gold Star (carbonate)
40. Magnesium Carbonate [usan]
41. Australian Magnesite
42. Hsdb 211
43. Einecs 208-915-9
44. Nsc 83511
45. Epa Pesticide Chemical Code 073503
46. Ci 77713
47. Ai3-00768
48. Magnesiumkarbonat
49. Unii-0ihc698356
50. Einecs 231-817-2
51. Carbonic Acid, Magnesium Salt (1:?)
52. Mgco3
53. Magnesium Carbonate Usp
54. Ec 208-915-9
55. Magnesium Carbonate, Usp Grade
56. Chembl1200736
57. Dtxsid4049660
58. Magnesium Carbonate Microparticles
59. Cs-b1764
60. Magnesium Carbonate [who-dd]
61. Akos015903527
62. Db09481
63. E504
64. Anhydrous Magnesium Carbonate [mart.]
65. Ft-0774766
66. Q407931
67. Cyclopentanecarboxylic Acid, 3-methyl-2-oxo-, Methyl Ester
68. 53678-75-4
| Molecular Weight | 84.31 g/mol |
|---|---|
| Molecular Formula | CMgO3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 83.9697855 g/mol |
| Monoisotopic Mass | 83.9697855 g/mol |
| Topological Polar Surface Area | 63.2 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 18.8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
THE CARBONATE ... /SALT/ OF MAGNESIUM /IS/ USED AS /ANTACID/, USUALLY IN COMBINATION WITH ALUMINUM HYDROXIDE.
American Medical Association, Council on Drugs. AMA Drug Evaluations Annual 1994. Chicago, IL: American Medical Association, 1994., p. 909
ALTHOUGH 1 G CONTAINS APPROX 20 MEQ ONLY FRACTION MAY BE AVAILABLE FOR NEUTRALIZATION IN VIVO. USUAL ANTACID DOSE OF 500 MG TO 2 G MAY BE INADEQUATE
Gilman, A. G., L. S. Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 6th ed. New York: Macmillan Publishing Co., Inc. 1980., p. 993
MEDICATION (VET): WHEN INSOL IN DIGESTIVE TRACT IT IS ANTIDIARRHEAL & COATS MUCOSAE; WHEN SLIGHTLY SOL FORM IS USED IT CAN BE AS LAXATIVE AS MAGNESIUM OXIDE. ... COMMERCIALLY AVAILABLE COSMETIC GRADES EXIST FOR RARE USE AS TOPICAL PROTECTANT, MOISTURE & FAT ABSORBENT.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 319
AS AN ANTACID, IT IS RELATIVELY WEAK (1 G NEUTRALIZES APPROX 7 ML OF 0.1 N HCL IN 10 MIN & 17 ML IN 2 HR).
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 735
For more Therapeutic Uses (Complete) data for MAGNESIUM CARBONATE (8 total), please visit the HSDB record page.
3= MODERATELY TOXIC: PROBABLE ORAL LETHAL DOSE (HUMAN) 0.5-5 G/KG; BETWEEN 1 OZ & 1 PINT (OR 1 LB) FOR 70 KG PERSON (150 LB).
Gosselin, R.E., R.P. Smith, H.C. Hodge. Clinical Toxicology of Commercial Products. 5th ed. Baltimore: Williams and Wilkins, 1984., p. II-127
Used as an over the counter antacid.
FDA Label
Neutralizes acid in the stomach.
Bleaching Agents
Chemicals that are used to oxidize pigments and thus effect whitening. (See all compounds classified as Bleaching Agents.)
Hygroscopic Agents
Materials that readily absorb moisture from their surroundings. (See all compounds classified as Hygroscopic Agents.)
A - Alimentary tract and metabolism
A02 - Drugs for acid related disorders
A02A - Antacids
A02AA - Magnesium compounds
A02AA01 - Magnesium carbonate
A - Alimentary tract and metabolism
A06 - Drugs for constipation
A06A - Drugs for constipation
A06AD - Osmotically acting laxatives
A06AD01 - Magnesium carbonate
Absorption
About 40-60% of magnesium is absorbed following oral administration. Percent absorption decreases as dose increases.
Route of Elimination
Primarily eliminated in urine.
Volume of Distribution
Vd for magnesium is 0.2-0.4L/kg. About 50% distributes to bone.
Clearance
Maximum magnesium clearance is directly proportional to creatinine clearance.
IN SHEEP TRIAL REAGENT GRADE MATERIAL DEMONSTRATED 72% TRUE ABSORPTION VALUES, WHILE COMMERCIAL MAGNESITE HAD ONLY 14% VALUE DRAMATIZING NEED FOR MORE BIOLOGIC AVAILABILITY STUDIES ON MANY FEED INGREDIENTS.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 319
Magnesium does not appear to be metabolized in any way.
Half life of 27.7 hours reported with overdose of 400mEq of magnesium in an adult.
Magnesium carbonate reacts with hydrochloric acid in the stomach to form carbon dioxide and magnesium chloride thus neutralizing excess acid in the stomach.
... Rapidly reacts with hydrochloric acid to form ... carbon dioxide /and magnesium chloride/.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 2774

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Dosage Form : Tablet
Grade : Oral
Application : Fillers, Diluents & Binders
Pharmacopoeia Ref : Conforms to USP-NF, Ph.Eur., J...
Technical Specs : Density- Tapped density- 440 g/l, Bulk density- 280 g/l; Particle size- D10- 25 ?m, D50- 60 ?m, D90-...
Ingredient(s) : Microcrystalline Cellulose
Dosage Form : Tablet
Grade : Oral
Application : Direct Compression
Pharmacopoeia Ref : Conforms to USP-NF, Ph.Eur., J...
Technical Specs : Density- Tapped density- 440 g/l, Bulk density- 310 g/l; Particle size- D10- 40 ?m, D50- 90 ?m, D90...
Ingredient(s) : Microcrystalline Cellulose
Dosage Form : Capsule
Grade : Oral
Application : Fillers, Diluents & Binders
Pharmacopoeia Ref : Conforms to USP-NF, Ph.Eur., J...
Technical Specs : Density- Tapped density- 857 g/l, Bulk density- 589 g/l; Particle size- D10- 5 ?m, D50- 40 ?m, D90-...
Ingredient(s) : Lactose Monohydrate
Dosage Form : Orodispersible Tablet
Grade : Oral
Application : Disintegrants & Superdisintegrants
Pharmacopoeia Ref : Conforms to USP-NF, Ph.Eur., J...
Technical Specs : Not Available
Ingredient(s) : Croscarmellose Sodium
Dosage Form : Capsule
Grade : Oral
Application : Disintegrants & Superdisintegrants
Excipient Details : Primojel® is a superdisintegrant suitable for a variety of tablet & capsule formulations. In higher concentrations, It can act as a dissolution enhancing agent.
Pharmacopoeia Ref : Conforms to USP-NF, Ph.Eur., J...
Technical Specs : Not Available
Ingredient(s) : Sodium Starch Glycolate
Dosage Form : Tablet
Grade : Oral
Application : Fillers, Diluents & Binders
Pharmacopoeia Ref : Conforms to USP-NF, Ph.Eur., J...
Technical Specs : Density- Tapped density- 716 g/l, Bulk density- 599 g/l; Particle size- D10-50 ?m, D50- 120 ?m, D90-...
Ingredient(s) : Lactose Monohydrate
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
85
PharmaCompass offers a list of Magnesium Carbonate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Magnesium Carbonate manufacturer or Magnesium Carbonate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Magnesium Carbonate manufacturer or Magnesium Carbonate supplier.
PharmaCompass also assists you with knowing the Magnesium Carbonate API Price utilized in the formulation of products. Magnesium Carbonate API Price is not always fixed or binding as the Magnesium Carbonate Price is obtained through a variety of data sources. The Magnesium Carbonate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Giobertite manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Giobertite, including repackagers and relabelers. The FDA regulates Giobertite manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Giobertite API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Giobertite manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Giobertite supplier is an individual or a company that provides Giobertite active pharmaceutical ingredient (API) or Giobertite finished formulations upon request. The Giobertite suppliers may include Giobertite API manufacturers, exporters, distributors and traders.
click here to find a list of Giobertite suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Giobertite DMF (Drug Master File) is a document detailing the whole manufacturing process of Giobertite active pharmaceutical ingredient (API) in detail. Different forms of Giobertite DMFs exist exist since differing nations have different regulations, such as Giobertite USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Giobertite DMF submitted to regulatory agencies in the US is known as a USDMF. Giobertite USDMF includes data on Giobertite's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Giobertite USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Giobertite suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Giobertite Drug Master File in Japan (Giobertite JDMF) empowers Giobertite API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Giobertite JDMF during the approval evaluation for pharmaceutical products. At the time of Giobertite JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Giobertite suppliers with JDMF on PharmaCompass.
A Giobertite CEP of the European Pharmacopoeia monograph is often referred to as a Giobertite Certificate of Suitability (COS). The purpose of a Giobertite CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Giobertite EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Giobertite to their clients by showing that a Giobertite CEP has been issued for it. The manufacturer submits a Giobertite CEP (COS) as part of the market authorization procedure, and it takes on the role of a Giobertite CEP holder for the record. Additionally, the data presented in the Giobertite CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Giobertite DMF.
A Giobertite CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Giobertite CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Giobertite suppliers with CEP (COS) on PharmaCompass.
A Giobertite written confirmation (Giobertite WC) is an official document issued by a regulatory agency to a Giobertite manufacturer, verifying that the manufacturing facility of a Giobertite active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Giobertite APIs or Giobertite finished pharmaceutical products to another nation, regulatory agencies frequently require a Giobertite WC (written confirmation) as part of the regulatory process.
click here to find a list of Giobertite suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Giobertite as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Giobertite API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Giobertite as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Giobertite and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Giobertite NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Giobertite suppliers with NDC on PharmaCompass.
Giobertite Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Giobertite GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Giobertite GMP manufacturer or Giobertite GMP API supplier for your needs.
A Giobertite CoA (Certificate of Analysis) is a formal document that attests to Giobertite's compliance with Giobertite specifications and serves as a tool for batch-level quality control.
Giobertite CoA mostly includes findings from lab analyses of a specific batch. For each Giobertite CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Giobertite may be tested according to a variety of international standards, such as European Pharmacopoeia (Giobertite EP), Giobertite JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Giobertite USP).
