
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
API
0
FDF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Weekly News Recap #Phispers
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
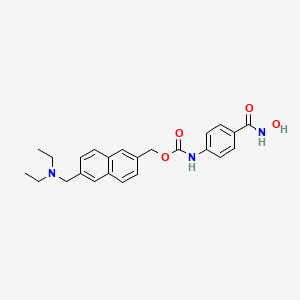

1. 497833-27-9
2. Itf2357
3. Givinostat [inn]
4. Givinostat Free Base
5. Itf-2357
6. [6-(diethylaminomethyl)naphthalen-2-yl]methyl N-[4-(hydroxycarbamoyl)phenyl]carbamate
7. 5p60f84fbh
8. Chembl1213492
9. 497833-27-9 (free Base)
10. (6-((diethylamino)methyl)naphthalen-2-yl)methyl (4-(hydroxycarbamoyl)phenyl)carbamate
11. (6-((diethylamino)methyl)naphthalen-2-yl)methyl(4-(hydroxycarbamoyl)phenyl)carbamate
12. [4-[(hydroxyamino)carbonyl]phenyl]carbamic Acid [6-[(diethylamino)methyl]-2-naphthalenyl]methyl Ester
13. Gavinostat
14. 4-[(hydroxyamino)carbonyl]phenyl]carbamic Acid [6-[(diethylamino)methyl]-2-naphthalenyl]methyl Ester
15. Unii-5p60f84fbh
16. {6-[(diethylamino)methyl]naphthalen-2-yl}methyl [4-(hydroxycarbamoyl)phenyl]carbamate
17. Gavinostat;itf-2357
18. Gtpl7490
19. Schembl1555814
20. Chebi:94187
21. Dtxsid70198049
22. Bcp29856
23. Zinc3820616
24. Bdbm50105329
25. Cs-4727
26. Db12645
27. Ex-5958
28. [6-(diethylaminomethyl)-2-naphthyl]methyl N-[4-(hydroxycarbamoyl)phenyl]carbamate
29. Ac-31380
30. Bcb04_000011
31. Hy-14842
32. Ec-000.2471
33. 833h279
34. A923985
35. Q426257
36. Gavinostat; Itf-2357; Itf2357; Itf 2357
37. Brd-k13810148-001-01-3
38. Carbamic Acid, (4-((hydroxyamino)carbonyl)phenyl)-, (6-((diethylamino)methyl)-2-naphthalenyl)methyl Ester
39. Qcm
| Molecular Weight | 421.5 g/mol |
|---|---|
| Molecular Formula | C24H27N3O4 |
| XLogP3 | 3.6 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 9 |
| Exact Mass | 421.20015635 g/mol |
| Monoisotopic Mass | 421.20015635 g/mol |
| Topological Polar Surface Area | 90.9 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 575 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Juvenile idiopathic arthritis
ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?