
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
US Medicaid
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
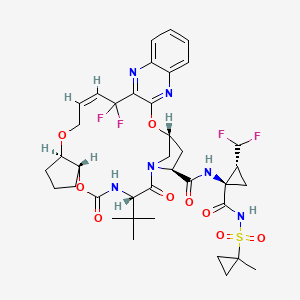

1. Abt-493
1. Abt-493
2. Glecaprevir [usan]
3. 1365970-03-1
4. A-1282576.0
5. K6buu8j72p
6. A-1282576
7. A-12825760
8. 1365970-03-1 (free)
9. Abt 493
10. (3ar,7s,10s,12r,21e,24ar)-7-tert-butyl-n-[(1r,2r)-2-(difluoromethyl)-1-{[(1-methylcyclopropyl)sulfonyl]carbamoyl}cyclopropyl]-20,20-difluoro-5,8-dioxo-2,3,3a,5,6,7,8,11,12,20,23,24a-dodecahydro-1h,10h-9,12-methanocyclopenta[18,19][1,10,17,3,6]trioxadiazacyclononadecino[11,12-b]quinoxaline-10-carboxamide
11. (3ar,7s,10s,12r,21e,24ar)-7-tert-butyl-n-{(1r,2r)-2(difluoromethyl)-1-[(1-methylcyclopropane-1-sulfonyl)carbamoyl]cyclopropyl}-20,20-difluoro5,8-dioxo-2,3,3a,5,6,7,8,11,12,20,23,24a-dodecahydro-1h,10h-9,12methanocyclopenta[18,19][1,10,17,3,6]trioxadiazacyclononadecino[11,12-b]quinoxaline-10carboxamide
12. Unii-k6buu8j72p
13. Maviret
14. (3ar,7s,10s,12r,21e,24ar)-7-tert-butyl-n-[(1r,2r)-2-(difluoromethyl)-1-{[(1-methylcyclopropyl)sulfonyl]carbamoyl}cyclop Ropyl]-20,20-difluoro-5,8-dioxo-2,3,3a,5,6,7,8,11,12,20,23,24a-dodecahydro-1h,10h-9,12-methanocyclopenta[18,19][1,10,17, 3,6]trioxadiazacyclononadecino[11,12-b]quinoxaline-10-carboxamide
15. O31
16. Glecaprevir [mi]
17. Abt-493(glecaprevir)
18. Glecaprevir [inn]
19. Glecaprevir (usan/inn)
20. Glecaprevir [who-dd]
21. Schembl883097
22. Abt493
23. Chembl3545363
24. Gtpl11267
25. Glecaprevir [orange Book]
26. Dtxsid901027945
27. (1r,14e,18r,22r,26s,29s)-26-tert-butyl-n-[(1r,2r)-2-(difluoromethyl)-1-[(1-methylcyclopropyl)sulfonylcarbamoyl]cyclopropyl]-13,13-difluoro-24,27-dioxo-2,17,23-trioxa-4,11,25,28-tetrazapentacyclo[26.2.1.03,12.05,10.018,22]hentriaconta-3,5,7,9,11,14-hexaene-29-carboxamide
28. Amy38157
29. Ex-a1940
30. Mavyret Component Glecaprevir
31. Bdbm50573891
32. S5720
33. Cs-8098
34. Db13879
35. Ac-33419
36. Hy-17634
37. J3.646.120i
38. D10814
39. 3,6]trioxadiazacyclononadecino[11,12-b]quinoxaline-10-carboxamide
40. (3ar,7s,10s,12r,21e,24ar)-7-tert-butyl-n-((1r,2r)-2-(difluoromethyl)-1-((1-methylcyclopropane-1-sulfonyl)carbamoyl)cyclopropyl)-20,20-difluoro-5,8-dioxo-2,3,3a,5,6,7,8,11,12,20,23,24a-dodecahydro-1h,10h-9,12-methanocyclopenta(18,19)(1,10,17,3,6)trioxadiazacyclononadecino(11,12-b)quinoxaline-10-carboxamide
41. (3ar,7s,10s,12r,21e,24ar)-7-tert-butyl-n-[(1r,2r)-2-(difluoromethyl)-1-{[(1-methylcyclopropyl)sulfonyl]carbamoyl}cyclop
42. (3ar,7s,10s,12r,21e,24ar)-7-tert-butyl-n-{(1r,2r)-2- (difluoromethyl)-1-[(1-methylcyclopropane-1-sulfonyl)carbamoyl]cyclopropyl}-20,20-difluoro- 5,8-dioxo-2,3,3a,5,6,7,8,11,12,20,23,24a-dodecahydro-1h,10h-9,12- Methanocyclopenta[18,19][1,10,17,3,6]trioxadiazacyclononadecino[11,12-b]quinoxaline-10- Carboxamide Hydrate
43. Cyclopropanecarboxamide, N-((((1r,2r)-2-((4,4-difluoro-4-(3-hydroxy-2-quinoxalinyl)-2-buten-1-yl)oxy)cyclopentyl)oxy)carbonyl)-3-methyl-l-valyl-(4r)-4-hydroxy-l-prolyl-1-amino-2-(difluoromethyl)-n-((1-methylcyclopropyl)sulfonyl)-, Cyclic (1->2)-ether, (1r,2r)-
44. Ropyl]-20,20-difluoro-5,8-dioxo-2,3,3a,5,6,7,8,11,12,20,23,24a-dodecahydro-1h,10h-9,12-methanocyclopenta[18,19][1,10,17,
| Molecular Weight | 838.9 g/mol |
|---|---|
| Molecular Formula | C38H46F4N6O9S |
| XLogP3 | 4.6 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 15 |
| Rotatable Bond Count | 7 |
| Exact Mass | 838.29831089 g/mol |
| Monoisotopic Mass | 838.29831089 g/mol |
| Topological Polar Surface Area | 204 Ų |
| Heavy Atom Count | 58 |
| Formal Charge | 0 |
| Complexity | 1760 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 7 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Indicated for the treatment of adult patients with chronic hepatitis C virus (HCV) genotype 1, 2, 3, 4, 5 or 6 infection without cirrhosis or with compensated cirrhosis (Child-Pugh A). MAVYRET is also indicated for the treatment of adult patients with HCV genotype 1 infection, who previously have been treated with a regimen containing an HCV NS5A inhibitor or an NS3/4A protease inhibitor (PI), but not both.
FDA Label
Maviret is indicated for the treatment of chronic hepatitis C virus (HCV) infection in adults and children aged 3 years and older.
Maviret coated granules is indicated for the treatment of chronic hepatitis C virus (HCV) infection in children 3 years and older.
In a biochemical assay studying clinical isolates of HCV genotypes 1a, 1b, 2a, 2b, 3a, 4a, 5a, and 6a, glecaprevir displayed IC50 values ranging from 3.5 to 11.3 nM that resulted in inhibition of the proteolytic activity of recombinant NS3/4A enzymes. In HCV replicon assays, glecaprevir had median EC50 values of 0.08-4.6 nM against laboratory and clinical isolates from subtypes 1a, 1b, 2a, 2b, 3a, 4a, 4d, 5a, and 6a. In a QT study, glecaprevir is not shown to prolong the QTc interval.
J05AP57
Absorption
In healthy subjects, the time it takes to reach the peak plasma concentration (Tmax) is approximately 5 hours. The mean peak plasma concentration (Cmax) is 597ng/mL in non-cirrhotic HCV-infected subjects. Relative to fasting conditions, the consumption of meals increases the absorption of glecaprevir by 83-163%.
Route of Elimination
The predominant route of elimination of the drug is biliary-fecal, where 92.1% of administered drug is excreted in feces and 0.7% of the drug is excreted in the urine.
Glecaprevir undergoes limited secondary metabolism in vitro, predominantly by CYP3A.
The elimination half life (t1/2) is approximately 6 hours.
Glecaprevir is an inhibitor of the HCV NS3/4A protease, which is a viral enzyme necessary for the proteolytic cleavage of the HCV encoded polyprotein into mature forms of the NS3, NS4A, NS4B, NS5A, and NS5B proteins. These multifunctional proteins, including NS3, are essential for viral replication. The N-terminal of NS3 protein confers serine protease activity, whileThe C-terminus of NS3 encodes a DExH/D-box RNA helicase which hydyolyzes NTP as an energy source to unwind double-stranded RNA in a 3 to 5 direction during replication of viral genomic RNA. NS4A is a cofactor for NS3 that directs the localization of NS3 and modulates its enzymatic activities. Glecaprevir disrupts the intracellular processes of the viral life cycle through inhibiting the NS3/4A protease activity of cleaving downstream junctions of HCV polypeptide and proteolytic processing of mature structural proteins.
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
53
PharmaCompass offers a list of Glecaprevir API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Glecaprevir manufacturer or Glecaprevir supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Glecaprevir manufacturer or Glecaprevir supplier.
PharmaCompass also assists you with knowing the Glecaprevir API Price utilized in the formulation of products. Glecaprevir API Price is not always fixed or binding as the Glecaprevir Price is obtained through a variety of data sources. The Glecaprevir Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Glecaprevir manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Glecaprevir, including repackagers and relabelers. The FDA regulates Glecaprevir manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Glecaprevir API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Glecaprevir manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Glecaprevir supplier is an individual or a company that provides Glecaprevir active pharmaceutical ingredient (API) or Glecaprevir finished formulations upon request. The Glecaprevir suppliers may include Glecaprevir API manufacturers, exporters, distributors and traders.
click here to find a list of Glecaprevir suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Glecaprevir Drug Master File in Korea (Glecaprevir KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Glecaprevir. The MFDS reviews the Glecaprevir KDMF as part of the drug registration process and uses the information provided in the Glecaprevir KDMF to evaluate the safety and efficacy of the drug.
After submitting a Glecaprevir KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Glecaprevir API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Glecaprevir suppliers with KDMF on PharmaCompass.
Glecaprevir Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Glecaprevir GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Glecaprevir GMP manufacturer or Glecaprevir GMP API supplier for your needs.
A Glecaprevir CoA (Certificate of Analysis) is a formal document that attests to Glecaprevir's compliance with Glecaprevir specifications and serves as a tool for batch-level quality control.
Glecaprevir CoA mostly includes findings from lab analyses of a specific batch. For each Glecaprevir CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Glecaprevir may be tested according to a variety of international standards, such as European Pharmacopoeia (Glecaprevir EP), Glecaprevir JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Glecaprevir USP).
