
Synopsis
Synopsis
0
USDMF
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book
0
Europe
0
Australia
0
South Africa
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz

1. Cidex
2. Diswart
3. Gludesin
4. Glutaral
5. Glutardialdehyde
6. Glutarol
7. Korsolex
8. Novaruca
9. Sekumatic
10. Sonacide
11. Sporicidin
1. Pentanedial
2. Glutaral
3. 111-30-8
4. Glutaric Dialdehyde
5. Cidex
6. 1,5-pentanedial
7. Sonacide
8. Glutardialdehyde
9. Pentane-1,5-dial
10. Glutaric Acid Dialdehyde
11. Glutaric Aldehyde
12. Glutaraldehyd
13. Glutaralum
14. Glutarol
15. Ucarcide
16. Aldesan
17. Alhydex
18. Hospex
19. 1,3-diformylpropane
20. Gluteraldehyde
21. 1,5-pentanedione
22. Aldesen
23. Novaruca
24. Sporicidin
25. Sterihyde L
26. Aldehyd Glutarowy
27. Nci-c55425
28. Glutaclean
29. Sterihyde
30. Aqucar
31. Veruca-sep
32. Relugan Gt
33. Relugan Gtw
34. Component Of Cidex
35. Glutarex 28
36. Nsc 13392
37. Sonacide (tn)
38. Cidex 7
39. Ucarcide 250
40. Relugan Gt 50
41. Sterihyde L (tn)
42. Coldcide-25 Microbiocide
43. Nsc-13392
44. Glutaral (jan/usp/inn)
45. Potentiated Acid Glutaraldehyde
46. Chebi:64276
47. T3c89m417n
48. 1, 5-pentanedial
49. Mfcd00007025
50. Ncgc00091110-01
51. Dsstox_cid_5355
52. Dsstox_rid_77761
53. Dsstox_gsid_25355
54. Glutaraldehyde Solution, 25%
55. Caswell No. 468
56. Glutaraldehyd [czech]
57. Glutaraldehyde Solution
58. 1,3-diformyl Propane
59. Diswart
60. Gludesin
61. Glutaralum [inn-latin]
62. Glutarol-1,5-pentanedial
63. Polyglutaraldehyde
64. Aldehyd Glutarowy [polish]
65. Glutaral [usan:inn:jan]
66. Cas-111-30-8
67. Poly(glutaraldehyde)
68. Ccris 3800
69. Hsdb 949
70. Einecs 203-856-5
71. Epa Pesticide Chemical Code 043901
72. Glutaric Dialdehyde Solution
73. Brn 0605390
74. Pentandial
75. Dioxopentane
76. Glutural
77. Ucarset
78. Verucasep
79. Glutaraldehyde Solution, For Electron Microscopy, ~25% In H2o
80. Virsal
81. Unii-t3c89m417n
82. Glutaral(usan)
83. Glutaric Dihydride
84. Glutaral [usan:usp:inn:jan]
85. Glutaraldehyde, 25% Soln
86. Glutaral Concentrate
87. Bactron K31
88. Ucarcide 225
89. Glutaraldehyde Solution (50% Or Less)
90. Pentanedial, Homopolymer
91. Glutaral [hsdb]
92. Glutaral [inci]
93. Glutaral [usan]
94. Pentane-1,5-dialdehyde
95. Glutaral [inn]
96. Glutaral [jan]
97. Glutaral, Inn, Usan
98. Glutaral [mart.]
99. Protectol Gda, Gt 50
100. Schembl836
101. Wln: Vh3vh
102. Glutaral [who-dd]
103. Ec 203-856-5
104. Glutaraldehyde [mi]
105. Pentane-1,5-dial Solution
106. Glutaraldehyde [fcc]
107. 4-01-00-03659 (beilstein Handbook Reference)
108. Bidd:er0299
109. Glutaraldehyde [vandf]
110. Glutaraldehyde Solution, 50%
111. Glutaral [usp Impurity]
112. Chembl1235482
113. Dtxsid6025355
114. Amy3308
115. Bio1_000462
116. Bio1_000951
117. Bio1_001440
118. Glutaraldehyde Solution, 25% W/w
119. Glutaraldehyde Solution, 50% W/w
120. Glutaraldehyde Solution, 70% W/w
121. Nsc13392
122. Str01121
123. Zinc1729593
124. Tox21_111083
125. Tox21_201742
126. Tox21_303295
127. Stl281872
128. Akos008967285
129. Glutaraldehyde (50per Cent In Water)
130. Db03266
131. Glutaric Dialdehyde, 25%sol. In Water
132. Glutaric Dialdehyde, 25% Sol. In Water
133. Ncgc00091110-02
134. Ncgc00091110-03
135. Ncgc00257231-01
136. Ncgc00259291-01
137. Glutaral Concentrate [usp Monograph]
138. Glutaraldehyde Solution, 25 Wt. % In H2o
139. Glutaraldehyde Solution, 50 Wt. % In H2o
140. Ft-0626730
141. G0067
142. G0068
143. En300-18037
144. D01120
145. Glutaraldehyde Solution, For Synthesis, 25.0%
146. Glutaraldehyde Solution, Grade Ii, 25% In H2o
147. A802339
148. Q416475
149. Glutaraldehyde Solution, For In Vitro Diagnostic Use
150. Q-201162
151. Glutaric Dialdehyde Solution, 50 Wt. % In H2o, Fcc
152. F2191-0161
153. Glutaraldehyde Solution, Saj First Grade, 20.0-26.0%
154. Glutaraldehyde Solution, Technical, ~25% In H2o (2.6 M)
155. Glutaraldehyde Solution, Technical, ~50% In H2o (5.6 M)
156. Glutaraldehyde Solution, 1.2 % (w/v) Glutaraldehyde In H2o
157. Glutaraldehyde Solution, For Electron Microscopy, ~50% In H2o
158. Glutaraldehyde Solution, For Electron Microscopy, ~8% In H2o
159. Glutaraldehyde Solution, 50% In H2o, Suitable For Photographic Applications
160. Glutaraldehyde Solution, Grade I, 25% In H2o, Specially Purified For Use As An Electron Microscopy Fixative
161. Glutaraldehyde Solution, Grade I, 50% In H2o, Specially Purified For Use As An Electron Microscopy Fixative Or Other Sophisticated Use
162. Glutaraldehyde Solution, Grade I, 70% In H2o, Specially Purified For Use As An Electron Microscopy Fixative Or Other Sophisticated Use
163. Glutaraldehyde Solution, Grade I, 8% In H2o, Specially Purified For Use As An Electron Microscopy Fixative Or Other Sophisticated Use
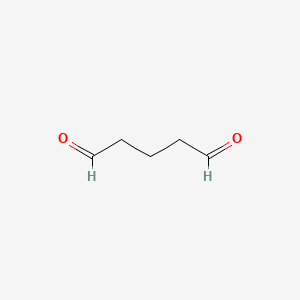
| Molecular Weight | 100.12 g/mol |
|---|---|
| Molecular Formula | C5H8O2 |
| XLogP3 | -0.5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 4 |
| Exact Mass | 100.052429494 g/mol |
| Monoisotopic Mass | 100.052429494 g/mol |
| Topological Polar Surface Area | 34.1 Ų |
| Heavy Atom Count | 7 |
| Formal Charge | 0 |
| Complexity | 51.1 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Disinfectants; Fixatives
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
VET: Disinfectants for foot-and-mouth disease were sprayed on livestock barns and roads from early February to May 2011. Although 90% of the disinfectant was concentrated on the roads, 10% was sprayed on cattle sheds and other sites where foot-and-mouth disease occurred. Since the outbreak of foot-and-mouth disease in November 2010, there has been a steady increase in disinfectant use. Consequently, its adverse environmental effects have prompted government officials to take preventive measures. The major chemical components of the disinfectants are citric acid, potassium sulfate base complex, quaternary ammonium compound, malic acid, and glutaraldehyde, ranging in amounts from tons to hundreds of tons. The exact amount of each component of the disinfectants could not be identified because the types of components used in the different commercial formulations overlapped. In this review, we obtained information on disinfectants that are widely used nationwide, including the types of major chemical components and their respective toxicities (both human and ecological).
PMID:23999336 Kim HM et al; J Infect Public Health 6(5):331-8 (2013)
For the topical treatment of warts, especially plantar warts.
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Glutarol (Last updated September 2005). Available from, as of July 9, 2010: https://www.medicines.org.uk/EMC/medicine/482/SPC/Glutarol+10%25+w+v+Cutaneous+Solution/
... A 43-year-old woman /developed/ an acute hemorrhagic colitis after colonoscopy with ambulatory anesthesia. The diagnosis is likely to have been glutaraldehyde induced colitis (used for disinfection of the endoscope). The patient recovered spontaneously completely.
PMID:15158241 Grenet M et al; Ann Fr Anesth Reanim 23 (5): 499-500 (2004).
Six eyes of 6 patients developed toxic anterior segment syndrome (TASS) after uneventful phacoemulsification cataract surgery with implantation of a 3-piece acrylic IOL performed by 2 ophthalmologists on the same day. Clinical findings included corneal edema, Descemet's membrane folds, anterior chamber reaction, fibrin formation, and irregular, dilated, and unreactive pupils. ... Glutaraldehyde 2% solution was used inadvertently by the operating room staff who cleaned and sterilized reusable ocular instruments before autoclaving. None of the affected corneas improved. Additional surgical procedures were required and included penetrating keratoplasty, trabeculectomy, and glaucoma tube implantation. CONCLUSIONS: Glutaraldehyde in concentrations generally used for cold sterilization is highly toxic to the corneal endothelium. The operating room staff involved in sterilizing instruments should be well educated about and careful to follow the protocols to properly clean and sterilize reusable ocular instruments.
PMID:17010870 Unal M et al; J Cataract Refract Surg 32 (10): 1696-701(2006).
The sequelae of the inadvertent introduction of glutaraldehyde into the peritoneal cavity /are documented/. ... The clinical course, progressive histological changes to the bowel at different periods over the course of 1 year, and what long-term morbidity remains /are described/. The chemical structure, effects, and pathogenesis of glutaraldehyde are described as well as suggestions for avoiding similar problems in the future.
PMID:16769324 Karpelowsky JS et al; J Pediatr Surg 41 (6): e23-5 (2006).
The medical records of patients with acute rectocolitis following endoscopy treated at Kaohsiung Veterans General Hospital since 2001 were reviewed. The indication of endoscopy was health check-up for all patients. Published English-language studies regarding acute rectocolitis following endoscopy were also reviewed. RESULTS: An outbreak of six patients occurred in April 2002 and one cirrhotic patient was admitted in July 2008. All patients developed a self-limited syndrome of abdominal pain and bloody diarrhea within 48 h of uncomplicated endoscopy. One severely ill patient required hospitalization to receive intravenous fluid and antibiotics. After the investigation in April 2002, glutaraldehyde-induced colitis was diagnosed due to a defect in the endoscope-cleansing procedure. There were no deficiencies in the cleansing procedure in July 2008. Considering the patient's concomitant disease, we postulated that ischemic colitis with cirrhosis-related intestinal inflammation and endotoxemia was the possible diagnosis in this sporadic case. CONCLUSIONS: Endoscopists should be aware of this iatrogenic complication in patients presenting with acute rectocolitis, especially in those who have undergone recent endoscopic examination. An outbreak of acute rectocolitis following endoscopy should be considered glutaraldehyde-induced and should lead to an investigation of cleansing and equipment-disinfection procedures. In the absence of strong evidence of an outbreak, an infectious disease, or contamination of glutaraldehyde, a sporadic case should be considered ischemic colitis especially in patients with relevant concomitant diseases or predisposing factors.
PMID:19636574 Hsu CW et al; Int J Colorectal Dis 24 (10): 1193-200 (2009)
For more Drug Warnings (Complete) data for Glutaraldehyde (9 total), please visit the HSDB record page.
3-4. 3= Moderately toxic, probable oral lethal dose (human) 0.5-5 g/kg, between 1 ounce & 1 pint for 70 kg person (150 lb). 4=Very toxic, probable oral lethal dose (human) 50-500 mg/kg, between 1 teaspoon and 1 ounce for 70 kg person (150 lb).
Gosselin, R.E., R.P. Smith, H.C. Hodge. Clinical Toxicology of Commercial Products. 5th ed. Baltimore: Williams and Wilkins, 1984., p. II-187
Disinfectants
Substances used on inanimate objects that destroy harmful microorganisms or inhibit their activity. Disinfectants are classed as complete, destroying SPORES as well as vegetative forms of microorganisms, or incomplete, destroying only vegetative forms of the organisms. They are distinguished from ANTISEPTICS, which are local anti-infective agents used on humans and other animals. (From Hawley's Condensed Chemical Dictionary, 11th ed) (See all compounds classified as Disinfectants.)
Fixatives
Agents employed in the preparation of histologic or pathologic specimens for the purpose of maintaining the existing form and structure of all of the constituent elements. Great numbers of different agents are used; some are also decalcifying and hardening agents. They must quickly kill and coagulate living tissue. (See all compounds classified as Fixatives.)
Cross-Linking Reagents
Reagents with two reactive groups, usually at opposite ends of the molecule, that are capable of reacting with and thereby forming bridges between side chains of amino acids in proteins; the locations of naturally reactive areas within proteins can thereby be identified; may also be used for other macromolecules, like glycoproteins, nucleic acids, or other. (See all compounds classified as Cross-Linking Reagents.)
Glutaraldehyde is readily absorbed by oral, iv, and inhalation routes. It is absorbed dermally to a lesser extent.
Dart, R.C. (ed). Medical Toxicology. Third Edition, Lippincott Williams & Wilkins. Philadelphia, PA. 2004., p. 1249
In rats, glutaraldehyde is eliminated in the urine and expired gases.
Dart, R.C. (ed). Medical Toxicology. Third Edition, Lippincott Williams & Wilkins. Philadelphia, PA. 2004., p. 1249
Dermal and intravenous studies in the rat with dilute aqueous glutaraldehyde solutions (0.075-7.5%) showed that, in dermal tests, approx 5% was absorbed in the rat, and 30-50% in the rabbit. In the intravenous injection tests, approx 12% was absorbed in the rat and approx 33% in the rabbit. There were no significant differences between males and females in the study. The dermal absorption rate constant was low (0.2-2 hr) in each species. The elimination times were long for both intravenous injection (t0.5 for the rat 10 hr, rabbit 15-30 hr) and dermal application (t0.5 for the rat 40-110 hr, rabbit 20-100 hr), possibly due to the binding of glutaraldehyde to protein and the slow excretion of metabolites. The principal metabolite in both species was CO2 with other metabolites not identified. /It was/ proposed that the metabolism probably involved initial oxidation to corresponding carboxylic acids by aldehyde dehydrogenase, and then further oxidation to CO2.
Organization for Economic Cooperation and Development; Screening Information Data Set for Glutaraldehyde, CAS 111-30-8 p.67 (October 1998). Available from, as of June 1, 2010: https://www.chem.unep.ch/irptc/sids/OECDSIDS/sidspub.html
In vitro studies using human skin tissue showed that glutaraldehyde did not penetrate the thick skin of the sole, but 3-14% penetrated the stratum corneum of the chest and abdomen and 3-4% penetrated the epidermis.
Organization for Economic Cooperation and Development; Screening Information Data Set for Glutaraldehyde, CAS 111-30-8 p.67 (October 1998). Available from, as of June 1, 2010: https://www.chem.unep.ch/irptc/sids/OECDSIDS/sidspub.html
Material balance and pharmacokinetic studies were conducted with rats & rabbits including iv or topical dosing with [14C]glutaraldehyde. Intravenous dosing resulted in radiochemical recovery from 86% to 101%. Principal route of recovery was as CO2 at 22% to 80% of the administered dose (7%-28% urinary, 0.2%-5% feces). Epicutaneous dosing resutled in radiochemical recovery primarily in the skin at the site of application (31%-61%) with no consistent accumulation in any other tissue. Rabbits absorbed 33% to 53% of the epicutaneously administered dose & rats absorbed 4.1% to 8.7%. Pharmacokinetic studies indicated percutaneous radiochemical absorption of 0.3% to 2.1% for rats & 2.5% to 15.6% for rabbits under conservative study conditions that are likely to overestimate potential human exposure conditions.
American Conference of Governmental Industrial Hygienists. Documentation of the TLVs and BEIs with Other World Wide Occupational Exposure Values. 7th Ed. CD-ROM Cincinnati, OH 45240-1634 2013., p. 4
Glutaraldehyde is either metabolized by aldehyde dehydrogenase to yield an acid or inactivated by a reaction with sulphydryl groups using glutathione.
Dart, R.C. (ed). Medical Toxicology. Third Edition, Lippincott Williams & Wilkins. Philadelphia, PA. 2004., p. 1249
... In the intravenous injection tests, approx 12% was absorbed in the rat and approx 33% in the rabbit ... The principal metabolite in both species was CO2 with other metabolites not identified. /It was/ proposed that the metabolism probably involved initial oxidation to corresponding carboxylic acids by aldehyde dehydrogenase, and then further oxidation to CO2.
Organization for Economic Cooperation and Development; Screening Information Data Set for Glutaraldehyde, CAS 111-30-8 p.67 (October 1998). Available from, as of June 1, 2010: https://www.chem.unep.ch/irptc/sids/OECDSIDS/sidspub.html
... The probable major metabolic pathway /is considered to be/ initial oxidation to the corresponding mono- or dicarboxylic acid by aldehyde dehydrogenase and then further oxidation of the acidic intermediate to carbon dioxide.
American Conference of Governmental Industrial Hygienists. Documentation of the TLVs and BEIs with Other World Wide Occupational Exposure Values. 7th Ed. CD-ROM Cincinnati, OH 45240-1634 2013., p. 5
The elimination times were long for both intravenous injection (t0.5 for the rat 10 hr, rabbit 15-30 hr) and dermal application (t0.5 for the rat 40-110 hr, rabbit 20-100 hr), possibly due to the binding of glutaraldehyde to protein and the slow excretion of metabolites.
Organization for Economic Cooperation and Development; Screening Information Data Set for Glutaraldehyde, CAS 111-30-8 p.67 (October 1998). Available from, as of June 1, 2010: https://www.chem.unep.ch/irptc/sids/OECDSIDS/sidspub.html

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Market Place

ABOUT THIS PAGE
80
PharmaCompass offers a list of Glutaraldehyde API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Glutaraldehyde manufacturer or Glutaraldehyde supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Glutaraldehyde manufacturer or Glutaraldehyde supplier.
PharmaCompass also assists you with knowing the Glutaraldehyde API Price utilized in the formulation of products. Glutaraldehyde API Price is not always fixed or binding as the Glutaraldehyde Price is obtained through a variety of data sources. The Glutaraldehyde Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Glutaraldehyde manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Glutaraldehyde, including repackagers and relabelers. The FDA regulates Glutaraldehyde manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Glutaraldehyde API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Glutaraldehyde manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Glutaraldehyde supplier is an individual or a company that provides Glutaraldehyde active pharmaceutical ingredient (API) or Glutaraldehyde finished formulations upon request. The Glutaraldehyde suppliers may include Glutaraldehyde API manufacturers, exporters, distributors and traders.
click here to find a list of Glutaraldehyde suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Glutaraldehyde CEP of the European Pharmacopoeia monograph is often referred to as a Glutaraldehyde Certificate of Suitability (COS). The purpose of a Glutaraldehyde CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Glutaraldehyde EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Glutaraldehyde to their clients by showing that a Glutaraldehyde CEP has been issued for it. The manufacturer submits a Glutaraldehyde CEP (COS) as part of the market authorization procedure, and it takes on the role of a Glutaraldehyde CEP holder for the record. Additionally, the data presented in the Glutaraldehyde CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Glutaraldehyde DMF.
A Glutaraldehyde CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Glutaraldehyde CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Glutaraldehyde suppliers with CEP (COS) on PharmaCompass.
Glutaraldehyde Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Glutaraldehyde GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Glutaraldehyde GMP manufacturer or Glutaraldehyde GMP API supplier for your needs.
A Glutaraldehyde CoA (Certificate of Analysis) is a formal document that attests to Glutaraldehyde's compliance with Glutaraldehyde specifications and serves as a tool for batch-level quality control.
Glutaraldehyde CoA mostly includes findings from lab analyses of a specific batch. For each Glutaraldehyde CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Glutaraldehyde may be tested according to a variety of international standards, such as European Pharmacopoeia (Glutaraldehyde EP), Glutaraldehyde JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Glutaraldehyde USP).

