
Synopsis
Synopsis
0
VMF
0
Weekly News Recap #Phispers
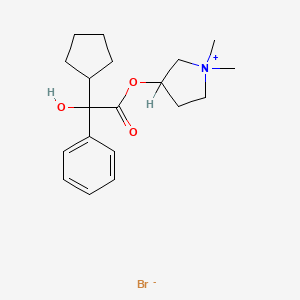

1. Bromide, Glycopyrronium
2. Glycopyrronium
3. Glycopyrronium Bromide
4. Nva 237
5. Nva-237
6. Nva237
7. Pyrrolidinium, 3-((cyclopentylhydroxyphenylacetyl)oxy)-1,1-dimethyl-, Bromide
1. 596-51-0
2. Glycopyrrolate Bromide
3. Robinul
4. Glycopyrronium Bromide
5. Gastrodyn
6. Nodapton
7. Tarodyl
8. Tarodyn
9. Asecryl
10. Cuvposa
11. Glycopyrronii Bromidum
12. Robanul
13. Ahr-504
14. Bromuro De Glicopirronio
15. Bromure De Glycopyrronium
16. Nva-237
17. Robinal
18. Seebri Neohaler
19. 3-hydroxy-1,1-dimethylpyrrolidinium Bromide Alpha-cyclopentylmandelate
20. Seebri
21. 1,1-dimethyl-3-hydroxypyrrolidinium Bromide Alpha-cyclopentylmandelate
22. 1-methyl-3-pyrrolidyl Alpha-phenylcyclopentaneglycolate Methobromide
23. 3-(2-cyclopentyl-2-hydroxy-2-phenylacetoxy)-1,1-dimethylpyrrolidin-1-ium Bromide
24. Pyrrolidinium, 3-[(cyclopentylhydroxyphenylacetyl)oxy]-1,1-dimethyl-,bromide
25. Ad-237
26. Pt-001
27. Nsc-250836
28. Nsc-251251
29. Nsc-251252
30. Copyrrolate
31. Dsstox_cid_3109
32. 3-(2-cyclopentyl-2-hydroxy-2-phenylacetoxy)-1,1-dimethylpyrrolidinium Bromide
33. (1,1-dimethylpyrrolidin-1-ium-3-yl) 2-cyclopentyl-2-hydroxy-2-phenylacetate Bromide
34. Dsstox_rid_76878
35. Dsstox_gsid_23109
36. Glycopyrronium (bromide);glycopyrrolate (bromide)
37. Nva237
38. Robinul Forte
39. Pyrrolidinium,1-dimethyl-, Bromide
40. Seebri Breezhaler
41. 53808-86-9
42. (1,1-dimethylpyrrolidin-1-ium-3-yl) 2-cyclopentyl-2-hydroxy-2-phenylacetate;bromide
43. Smr000469282
44. Glycopyrrolate, Erythro-
45. Nsc 250836
46. Nsc 251251
47. Nsc 251252
48. Org-nc-45
49. Nsc250836
50. Nsc251251
51. Nsc251252
52. (2s,3's)-glycopyrrolate
53. Ahr 504
54. Glycopyrronium (as Bromide)
55. Nva 237
56. Glycopyrronii Bromidum [inn-latin]
57. Ncgc00179456-02
58. Wln: T5ktj A1 A1 Covxqr&- Al5tj &q &e
59. Einecs 209-887-0
60. Mfcd00072137
61. Bromure De Glycopyrronium [inn-french]
62. Bromuro De Glicopirronio [inn-spanish]
63. Chf5259
64. Glycopyrrolate [usan:usp]
65. Pt001
66. Chf-5259
67. Lonhala Magnair
68. Pyrrolidinium,1-dimethyl-, Bromide, .alpha.-cyclopentylmandelate
69. 1,1-dimethyl-3-hydroxypyrrolidinium Bromide .alpha.-cyclopentylmandelate
70. 1-methyl-3-pyrrolidinyl .alpha.-phenylcyclopentaneglycolate Methobromide
71. 3-hydroxy-1,1-dimethylpyrrolidinium Bromide-.alpha.-cyclopentylmandelate
72. Mandelic Acid, Ester With 3-hydroxy-1,1-dimethylpyrrolidinium Bromide
73. Cuvposa (tn)
74. Robinul (tn)
75. Glycopyrrolate (usp)
76. .beta.-1-methyl-3-pyrrolidyl-.alpha.-cyclopentylmandelate Methobromide
77. Seebri Breezhaler (tn)
78. Cas-596-51-0
79. Glycopyrrone Bromide
80. Schembl41436
81. Glycopyrronium Bromide ,(s)
82. Mls001424112
83. Mls002222301
84. 3-hydroxy-1,1-dimethylpyrrolidinium Bromide .alpha.-cyclopentylmandelate
85. Chembl1201027
86. Dtxsid6023109
87. Chebi:90972
88. Glycopyrrolate, >=98% (hplc)
89. Glycopyrronium Bromide (jan/inn)
90. Hms1570e14
91. Hms2051p12
92. Hms2094a05
93. Hms2097e14
94. Hms2235f12
95. Hms3259p04
96. Hms3369f10
97. Hms3393p12
98. Hms3714e14
99. Hms3885p14
100. Pharmakon1600-01505753
101. Amy22352
102. Bcp07110
103. Bcp33298
104. Chf-5992
105. Ex-a4155
106. Tox21_113144
107. Tox21_113145
108. Nsc759238
109. S4660
110. Akos015962136
111. Pyrrolidinium, 1,1-dimethyl-3-hydroxy-, Bromide, Alpha-cyclopentylmandelate
112. Pyrrolidinium, 3-hydroxy-1,1-dimethyl-, Bromide, Alpha-cyclopentylmandelate
113. Tox21_113144_1
114. Ccg-101030
115. Ccg-213543
116. Cs-1763
117. Nc00280
118. Nc00694
119. Nsc-759238
120. Ncgc00179456-04
121. Ac-23382
122. Hy-17465
123. Glycopyrrolate Erythro Isomer (ss-isomer)
124. Ft-0626787
125. G0392
126. D00540
127. A832400
128. Sr-01000763650
129. Sr-01000763650-3
130. Q27162963
131. Glycopyrrolate Erythro Isomer (mixture Of Rr-isomer And Ss-isomer)
132. Glycopyrrolate, United States Pharmacopeia (usp) Reference Standard
133. Glycopyrronium Bromide, European Pharmacopoeia (ep) Reference Standard
134. Glycopyrronium Impurity N, European Pharmacopoeia (ep) Reference Standard
135. 3-(2-cyclopentyl-2-hydroxy-2-phenylacetoxy)-1,1-dimethylpyrrolidin-1-iumbromide
136. 3-{[cyclopentyl(hydroxy)phenylacetyl]oxy}-1,1-dimethylpyrrolidin-1-ium Bromide
137. Glycopyrronium For Peak Identification, European Pharmacopoeia (ep) Reference Standard
| Molecular Weight | 398.3 g/mol |
|---|---|
| Molecular Formula | C19H28BrNO3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 5 |
| Exact Mass | 397.12526 g/mol |
| Monoisotopic Mass | 397.12526 g/mol |
| Topological Polar Surface Area | 46.5 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 424 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 2 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 8 | |
|---|---|
| Drug Name | Cuvposa |
| PubMed Health | Glycopyrrolate |
| Drug Classes | Cholinergic Antagonist, Gastrointestinal Agent |
| Drug Label | CUVPOSA is an anticholinergic drug available as an oral solution containing 1 mg glycopyrrolate per 5 mL. The chemical name for glycopyrrolate is pyrrolidinium, 3-[(cyclopentylhydroxyphenylacetyl) oxy]-1,1-dimethyl-,bromide. The chemical structure is... |
| Active Ingredient | Glycopyrrolate |
| Dosage Form | Solution |
| Route | Oral |
| Strength | 1mg/5ml |
| Market Status | Prescription |
| Company | Merz Pharms |
| 2 of 8 | |
|---|---|
| Drug Name | Glycopyrrolate |
| PubMed Health | Glycopyrrolate |
| Drug Classes | Cholinergic Antagonist, Gastrointestinal Agent |
| Drug Label | Glycopyrrolate tablets contain the synthetic anticholinergic glycopyrrolate. Glycopyrrolate is a quaternary ammonium compound with the following chemical name:3-[(cyclopentylhydroxyphenylacetyl)oxy]-1,1-dimethylpyrrolidinium bromide. Its empirical fo... |
| Active Ingredient | Glycopyrrolate |
| Dosage Form | Tablet; Injectable |
| Route | Injection; Oral |
| Strength | 1mg; 0.2mg/ml; 1.5mg; 2mg |
| Market Status | Prescription |
| Company | Stason Pharms; Vintage Pharms; Ranbaxy; Aurolife Pharma; Excellium; Hikma Farmaceutica; Par Pharm; Vintage; Luitpold; Dr Reddys Labs; Nexgen Pharma |
| 3 of 8 | |
|---|---|
| Drug Name | Robinul |
| PubMed Health | Glycopyrrolate |
| Drug Classes | Cholinergic Antagonist, Gastrointestinal Agent |
| Drug Label | ROBINUL (glycopyrrolate) Injection is a synthetic anticholinergic agent. Each 1 mL contains:Glycopyrrolate, USP 0.2 mgWater for Injection, USP q.s.Benzyl Alcohol, NF 0.9% (preservative)pH adjusted, when necessary, with hydrochloric acid and/or sodium... |
| Active Ingredient | Glycopyrrolate |
| Dosage Form | Tablet; Injectable |
| Route | Injection; Oral |
| Strength | 1mg; 0.2mg/ml |
| Market Status | Prescription |
| Company | Shionogi; Hikma Maple |
| 4 of 8 | |
|---|---|
| Drug Name | Robinul forte |
| PubMed Health | Glycopyrrolate (By mouth) |
| Drug Classes | Cholinergic Antagonist, Gastrointestinal Agent |
| Drug Label | Robinul and Robinul Forte tablets contain the synthetic anticholinergic, glycopyrrolate. Glycopyrrolate is a quaternary ammonium compound with the following chemical name: 3-[(cyclopentylhydroxyphenylacetyl)oxy]-1, 1-dimethylpyrrolidinium bromide... |
| Active Ingredient | Glycopyrrolate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 2mg |
| Market Status | Prescription |
| Company | Shionogi |
| 5 of 8 | |
|---|---|
| Drug Name | Cuvposa |
| PubMed Health | Glycopyrrolate |
| Drug Classes | Cholinergic Antagonist, Gastrointestinal Agent |
| Drug Label | CUVPOSA is an anticholinergic drug available as an oral solution containing 1 mg glycopyrrolate per 5 mL. The chemical name for glycopyrrolate is pyrrolidinium, 3-[(cyclopentylhydroxyphenylacetyl) oxy]-1,1-dimethyl-,bromide. The chemical structure is... |
| Active Ingredient | Glycopyrrolate |
| Dosage Form | Solution |
| Route | Oral |
| Strength | 1mg/5ml |
| Market Status | Prescription |
| Company | Merz Pharms |
| 6 of 8 | |
|---|---|
| Drug Name | Glycopyrrolate |
| PubMed Health | Glycopyrrolate |
| Drug Classes | Cholinergic Antagonist, Gastrointestinal Agent |
| Drug Label | Glycopyrrolate tablets contain the synthetic anticholinergic glycopyrrolate. Glycopyrrolate is a quaternary ammonium compound with the following chemical name:3-[(cyclopentylhydroxyphenylacetyl)oxy]-1,1-dimethylpyrrolidinium bromide. Its empirical fo... |
| Active Ingredient | Glycopyrrolate |
| Dosage Form | Tablet; Injectable |
| Route | Injection; Oral |
| Strength | 1mg; 0.2mg/ml; 1.5mg; 2mg |
| Market Status | Prescription |
| Company | Stason Pharms; Vintage Pharms; Ranbaxy; Aurolife Pharma; Excellium; Hikma Farmaceutica; Par Pharm; Vintage; Luitpold; Dr Reddys Labs; Nexgen Pharma |
| 7 of 8 | |
|---|---|
| Drug Name | Robinul |
| PubMed Health | Glycopyrrolate |
| Drug Classes | Cholinergic Antagonist, Gastrointestinal Agent |
| Drug Label | ROBINUL (glycopyrrolate) Injection is a synthetic anticholinergic agent. Each 1 mL contains:Glycopyrrolate, USP 0.2 mgWater for Injection, USP q.s.Benzyl Alcohol, NF 0.9% (preservative)pH adjusted, when necessary, with hydrochloric acid and/or sodium... |
| Active Ingredient | Glycopyrrolate |
| Dosage Form | Tablet; Injectable |
| Route | Injection; Oral |
| Strength | 1mg; 0.2mg/ml |
| Market Status | Prescription |
| Company | Shionogi; Hikma Maple |
| 8 of 8 | |
|---|---|
| Drug Name | Robinul forte |
| PubMed Health | Glycopyrrolate (By mouth) |
| Drug Classes | Cholinergic Antagonist, Gastrointestinal Agent |
| Drug Label | Robinul and Robinul Forte tablets contain the synthetic anticholinergic, glycopyrrolate. Glycopyrrolate is a quaternary ammonium compound with the following chemical name: 3-[(cyclopentylhydroxyphenylacetyl)oxy]-1, 1-dimethylpyrrolidinium bromide... |
| Active Ingredient | Glycopyrrolate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 2mg |
| Market Status | Prescription |
| Company | Shionogi |
Tovanor Breezhaler is indicated as a maintenance bronchodilator treatment to relieve symptoms in adult patients with chronic obstructive pulmonary disease (COPD).
Seebri Breezhaler is indicated as a maintenance bronchodilator treatment to relieve symptoms in adult patients with chronic obstructive pulmonary disease (COPD).
Enurev Breezhaler is indicated as a maintenance bronchodilator treatment to relieve symptoms in adult patients with chronic obstructive pulmonary disease (COPD).
Symptomatic treatment of severe sialorrhoea (chronic pathological drooling) in children and adolescents aged 3 years and older with chronic neurological disorders.
Treatment of sialorrhoea
Chronic obstructive pulmonary disease
Treatment of sialorrhoea
Treatment of hyperhidrosis
Muscarinic Antagonists
Drugs that bind to but do not activate MUSCARINIC RECEPTORS, thereby blocking the actions of endogenous ACETYLCHOLINE or exogenous agonists. Muscarinic antagonists have widespread effects including actions on the iris and ciliary muscle of the eye, the heart and blood vessels, secretions of the respiratory tract, GI system, and salivary glands, GI motility, urinary bladder tone, and the central nervous system. (See all compounds classified as Muscarinic Antagonists.)
Adjuvants, Anesthesia
Agents that are administered in association with anesthetics to increase effectiveness, improve delivery, or decrease required dosage. (See all compounds classified as Adjuvants, Anesthesia.)
R03BB06
R03BB06
R03BB06
A03AB02
A03AB02
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
A - Alimentary tract and metabolism
A03 - Drugs for functional gastrointestinal disorders
A03A - Drugs for functional gastrointestinal disorders
A03AB - Synthetic anticholinergics, quaternary ammonium compounds
A03AB02 - Glycopyrronium bromide
R - Respiratory system
R03 - Drugs for obstructive airway diseases
R03B - Other drugs for obstructive airway diseases, inhalants
R03BB - Anticholinergics
R03BB06 - Glycopyrronium bromide

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
QVM149 combines the bronchodilation of indacaterol acetate (a LABA) and the antimuscarinic effects of glycopyrronium bromide (a LAMA) with mometasone furoate in a precise once-daily formulation, delivered via the dose-confirming Breezhaler® device.
Lead Product(s): Indacaterol Acetate,Glycopyrronium Bromide,Mometasone Furoate
Therapeutic Area: Pulmonary/Respiratory Diseases Brand Name: Enerzair Breezhaler
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Novartis Pharmaceuticals Corporation
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable July 07, 2020
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Indacaterol Acetate,Glycopyrronium Bromide,Mometasone Furoate
Therapeutic Area : Pulmonary/Respiratory Diseases
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Novartis Pharmaceuticals Corporation
Deal Size : Not Applicable
Deal Type : Not Applicable
QVM149 Receives Regulatory Approval in Europe and Japan
Details : QVM149 combines the bronchodilation of indacaterol acetate (a LABA) and the antimuscarinic effects of glycopyrronium bromide (a LAMA) with mometasone furoate in a precise once-daily formulation, delivered via the dose-confirming Breezhaler® device.
Brand Name : Enerzair Breezhaler
Molecule Type : Small molecule
Upfront Cash : Not Applicable
July 07, 2020
Details:
Cuvposa is an anticholinergic indicated to reduce chronic severe drooling in patients aged 3-16 years with neurologic conditions associated with problem drooling.
Lead Product(s): Glycopyrronium Bromide
Therapeutic Area: Neurology Brand Name: Cuvposa-Generic
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable August 20, 2024
Lead Product(s) : Glycopyrronium Bromide
Therapeutic Area : Neurology
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Granules India Gets USFDA Nod for Generic Glycopyrrolate Oral Solution
Details : Cuvposa is an anticholinergic indicated to reduce chronic severe drooling in patients aged 3-16 years with neurologic conditions associated with problem drooling.
Brand Name : Cuvposa-Generic
Molecule Type : Small molecule
Upfront Cash : Not Applicable
August 20, 2024
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Vilfuro-G (vilanterol, fluticasone furoate & glycopyrronium bromide) is world's first fixed-dose triple combination drug which is approved for the treatment of chronic obstructive pulmonary disease (COPD).
Lead Product(s): Vilanterol Trifenatate,Fluticasone Furoate,Glycopyrronium Bromide
Therapeutic Area: Pulmonary/Respiratory Diseases Brand Name: Vilfuro-G
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable November 23, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Vilanterol Trifenatate,Fluticasone Furoate,Glycopyrronium Bromide
Therapeutic Area : Pulmonary/Respiratory Diseases
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Lupin Launches World’s First Fixed-dose Triple Combination Drug, Vilfuro-G® for COPD Management...
Details : Vilfuro-G (vilanterol, fluticasone furoate & glycopyrronium bromide) is world's first fixed-dose triple combination drug which is approved for the treatment of chronic obstructive pulmonary disease (COPD).
Brand Name : Vilfuro-G
Molecule Type : Small molecule
Upfront Cash : Not Applicable
November 23, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
A fixed dose combination of vilanterol (adrenergic receptor beta-2 agonist), glycopyrrolate & fluticasone furoate is approved for the treatment of chronic obstructive pulmonary disease.
Lead Product(s): Vilanterol Trifenatate,Glycopyrronium Bromide,Fluticasone Furoate
Therapeutic Area: Pulmonary/Respiratory Diseases Brand Name: Undisclosed
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable October 13, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Vilanterol Trifenatate,Glycopyrronium Bromide,Fluticasone Furoate
Therapeutic Area : Pulmonary/Respiratory Diseases
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : A fixed dose combination of vilanterol (adrenergic receptor beta-2 agonist), glycopyrrolate & fluticasone furoate is approved for the treatment of chronic obstructive pulmonary disease.
Brand Name : Undisclosed
Molecule Type : Small molecule
Upfront Cash : Not Applicable
October 13, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
PREVDUO (neostigmine methylsulfate and glycopyrrolate injection) pre-filled syringe, the first and only FDA-approved neostigmine–glycopyrrolate combination product in the U.S for Neuromuscular blockade.
Lead Product(s): Neostigmine Methylsulfate,Glycopyrronium Bromide
Therapeutic Area: Neurology Brand Name: Prevduo
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable June 12, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Neostigmine Methylsulfate,Glycopyrronium Bromide
Therapeutic Area : Neurology
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : PREVDUO (neostigmine methylsulfate and glycopyrrolate injection) pre-filled syringe, the first and only FDA-approved neostigmine–glycopyrrolate combination product in the U.S for Neuromuscular blockade.
Brand Name : Prevduo
Molecule Type : Small molecule
Upfront Cash : Not Applicable
June 12, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Glycopyrrolate is an anticholinergic agents, competitively inhibit binding of the neurotransmitter, acetylcholine. Thus, it diminishes the volume and free acidity of gastric secretions and controls excessive pharyngeal, tracheal, and bronchial secretions.
Lead Product(s): Glycopyrronium Bromide
Therapeutic Area: Gastroenterology Brand Name: Glycopyrrolate-Generic
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable February 09, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Glycopyrronium Bromide
Therapeutic Area : Gastroenterology
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Lupin Receives Approval From USFDA For Glycopyrrolate Injection USP
Details : Glycopyrrolate is an anticholinergic agents, competitively inhibit binding of the neurotransmitter, acetylcholine. Thus, it diminishes the volume and free acidity of gastric secretions and controls excessive pharyngeal, tracheal, and bronchial secretions...
Brand Name : Glycopyrrolate-Generic
Molecule Type : Small molecule
Upfront Cash : Not Applicable
February 09, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Glycopyrrolate injection is used as a pre-operative medication to inhibit salivary gland and respiratory secretions also as adjunctive therapy for the treatment of peptic ulcer when rapid anticholinergic effect is desired or when oral medication is not tolerated.
Lead Product(s): Glycopyrronium Bromide
Therapeutic Area: Neurology Brand Name: Glycopyrrolate-Generic
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable November 01, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Glycopyrronium Bromide
Therapeutic Area : Neurology
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Alembic Pharma Gets USFDA Nod For Generic Version Of Glycopyrrolate Injection
Details : Glycopyrrolate injection is used as a pre-operative medication to inhibit salivary gland and respiratory secretions also as adjunctive therapy for the treatment of peptic ulcer when rapid anticholinergic effect is desired or when oral medication is not t...
Brand Name : Glycopyrrolate-Generic
Molecule Type : Small molecule
Upfront Cash : Not Applicable
November 01, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Glycopyrrolate Injection, USP 0.6 mg per 3 mL Simplist prefilled syringe may be administered prior to or concomitantly with Simplist Neostigmine Methylsulfate Injection, USP 3 mg per 3 mL prefilled syringe for reversal of neuromuscular blockade.
Lead Product(s): Glycopyrronium Bromide
Therapeutic Area: Neurology Brand Name: Glycopyrrolate-Generic
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable July 25, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Glycopyrronium Bromide
Therapeutic Area : Neurology
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Fresenius Kabi Expands Ready-to-Administer Portfolio with Glycopyrrolate Injection, USP Simplist®...
Details : Glycopyrrolate Injection, USP 0.6 mg per 3 mL Simplist prefilled syringe may be administered prior to or concomitantly with Simplist Neostigmine Methylsulfate Injection, USP 3 mg per 3 mL prefilled syringe for reversal of neuromuscular blockade.
Brand Name : Glycopyrrolate-Generic
Molecule Type : Small molecule
Upfront Cash : Not Applicable
July 25, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
A recently completed Phase I clinical trial of the propellant HFO-1234ze in a pMDI containing budesonide, glycopyrronium, formoterol fumarate in healthy adults was positive, demonstrating similar safety and tolerability profile.
Lead Product(s): Budesonide,Glycopyrronium Bromide,Formoterol Fumarate
Therapeutic Area: Pulmonary/Respiratory Diseases Brand Name: Breztri Aerosphere
Study Phase: Phase IProduct Type: Small molecule
Sponsor: AstraZeneca
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Partnership February 22, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Budesonide,Glycopyrronium Bromide,Formoterol Fumarate
Therapeutic Area : Pulmonary/Respiratory Diseases
Highest Development Status : Phase I
Partner/Sponsor/Collaborator : AstraZeneca
Deal Size : Undisclosed
Deal Type : Partnership
Details : A recently completed Phase I clinical trial of the propellant HFO-1234ze in a pMDI containing budesonide, glycopyrronium, formoterol fumarate in healthy adults was positive, demonstrating similar safety and tolerability profile.
Brand Name : Breztri Aerosphere
Molecule Type : Small molecule
Upfront Cash : Undisclosed
February 22, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Covis has entered into an Exclusive Promotion and Distribution Agreement with Novartis Pharmaceuticals Canada, whereby Covis has been appointed as Novartis’ exclusive partner to promote and distribute PrSeebri® Breezhaler® and PrUltibro® Breezhaler® in Canada.
Lead Product(s): Glycopyrronium Bromide
Therapeutic Area: Pulmonary/Respiratory Diseases Brand Name: Seebri Breezhaler
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Novartis Pharmaceuticals Corporation
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Agreement January 05, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Glycopyrronium Bromide
Therapeutic Area : Pulmonary/Respiratory Diseases
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Novartis Pharmaceuticals Corporation
Deal Size : Undisclosed
Deal Type : Agreement
Covis Enters Promotion and Distribution Agreement for Two Respiratory Medicines, Seebri® Breezhal...
Details : Covis has entered into an Exclusive Promotion and Distribution Agreement with Novartis Pharmaceuticals Canada, whereby Covis has been appointed as Novartis’ exclusive partner to promote and distribute PrSeebri® Breezhaler® and PrUltibro® Breezhaler�...
Brand Name : Seebri Breezhaler
Molecule Type : Small molecule
Upfront Cash : Undisclosed
January 05, 2022

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
Patent Expiration Date : 2030-05-28
FORMOTEROL FUMARATE; GLYCOPYRROLATE
US Patent Number : 8324266
Drug Substance Claim :
Drug Product Claim :
Application Number : 208294
Patent Use Code : U-2889
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2030-05-28

Patent Expiration Date : 2030-05-28
FORMOTEROL FUMARATE; GLYCOPYRROLATE
US Patent Number : 8703806
Drug Substance Claim :
Drug Product Claim :
Application Number : 208294
Patent Use Code : U-2889
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2030-05-28

Patent Expiration Date : 2030-05-28
BUDESONIDE; FORMOTEROL FUMARATE; GLYCOPYRROLATE
US Patent Number : 9463161
Drug Substance Claim :
Drug Product Claim : Y
Application Number : 212122
Patent Use Code : U-2889
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2030-05-28

Patent Expiration Date : 2030-05-28
FORMOTEROL FUMARATE; GLYCOPYRROLATE
US Patent Number : 9415009
Drug Substance Claim :
Drug Product Claim :
Application Number : 208294
Patent Use Code : U-2889
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2030-05-28

Patent Expiration Date : 2030-05-28
BUDESONIDE; FORMOTEROL FUMARATE; GLYCOPYRROLATE
US Patent Number : 10716753
Drug Substance Claim :
Drug Product Claim : Y
Application Number : 212122
Patent Use Code : U-2889
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2030-05-28

Patent Expiration Date : 2030-05-28
FORMOTEROL FUMARATE; GLYCOPYRROLATE
US Patent Number : 10716753
Drug Substance Claim :
Drug Product Claim : Y
Application Number : 208294
Patent Use Code : U-2889
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2030-05-28

Patent Expiration Date : 2030-05-28
BUDESONIDE; FORMOTEROL FUMARATE; GLYCOPYRROLATE
US Patent Number : 9415009
Drug Substance Claim :
Drug Product Claim :
Application Number : 212122
Patent Use Code : U-2889
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2030-05-28

Patent Expiration Date : 2028-10-11
GLYCOPYRROLATE; INDACATEROL MALEATE
US Patent Number : 8479730
Drug Substance Claim :
Drug Product Claim : Y
Application Number : 207930
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2028-10-11

Patent Expiration Date : 2024-11-18
US Patent Number : 7458372
Drug Substance Claim :
Drug Product Claim : Y
Application Number : 208437
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2024-11-18

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?