
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
FDA Orange Book

0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Herpecin L
2. Herpecin-l
3. Herpecinl
4. Sebical
5. Woun'dres
1. 97-59-6
2. 5-ureidohydantoin
3. Glyoxyldiureide
4. 1-(2,5-dioxoimidazolidin-4-yl)urea
5. Cordianine
6. Allantol
7. Glyoxyldiureid
8. Sebical
9. Alantan
10. Avc/dienestrolcream
11. Urea, (2,5-dioxo-4-imidazolidinyl)-
12. Hydantoin, 5-ureido-
13. Psoralon
14. Septalan
15. Cutemol Emollient
16. Uniderm A
17. (2,5-dioxo-4-imidazolidinyl)urea
18. Glyoxylic(acid) Diureide
19. (2,5-dioxoimidazolidin-4-yl)urea
20. Glyoxylic Diureide
21. Nsc 7606
22. Caswell No. 024
23. Dl-allantoin
24. 5-ureido-2,4-imidazolidindion
25. N-(2,5-dioxo-4-imidazolidinyl)urea
26. Alwextin
27. Ccris 1958
28. 2,5-dioxo-4-imidazolidinyl-urea
29. Fancol Toin
30. 5-ureidohydrantoin
31. Epa Pesticide Chemical Code 085701
32. (+/-)-allantoin
33. 4-ureido-2,5-imidazolidinedione
34. Ai3-15281
35. Nsc7606
36. Allantoin (jan/usp)
37. Nsc-7606
38. Idelalisib Metabolite M1a
39. N-(2,5-dioxoimidazolidin-4-yl)urea
40. 97-59-6 (racemic)
41. Mls000737882
42. 5377-33-3
43. 5-ureido-2,4-imidazolidindione
44. Chebi:15676
45. 344s277g0z
46. Urea, N-(2,5-dioxo-4-imidazolidinyl)-
47. Dsstox_cid_43
48. Herpecin L
49. D00121
50. Dsstox_rid_75334
51. Dsstox_gsid_20043
52. Allantoin [usan:ban]
53. Smr000528073
54. Sr-01000766252
55. Einecs 202-592-8
56. Mfcd00005260
57. Brn 0102364
58. Unii-344s277g0z
59. Hsdb 7490
60. Allantoin [usan:usp:ban:jan]
61. Cas-97-59-6
62. Prestwick_11
63. Ncgc00016358-01
64. Allation,(s)
65. 5-ureido-hydantoin
66. Allantoin (8ci)
67. Spectrum_001078
68. Allantoin [jan]
69. Allantoin [ii]
70. Allantoin [mi]
71. Allantoin [hsdb]
72. Allantoin [inci]
73. Allantoin [usan]
74. Prestwick0_000002
75. Prestwick1_000002
76. Prestwick2_000002
77. Prestwick3_000002
78. Spectrum2_000219
79. Spectrum3_000876
80. Spectrum4_000716
81. Spectrum5_001526
82. Allantoin [vandf]
83. Allantoin [mart.]
84. Bmse000437
85. Ec 202-592-8
86. Allantoin [usp-rs]
87. Allantoin [who-dd]
88. Schembl3208
89. Oprea1_621175
90. Bspbio_000003
91. Bspbio_002551
92. Kbiogr_001271
93. Kbioss_001558
94. 5-25-15-00338 (beilstein Handbook Reference)
95. Mls002473300
96. Allantoin, Analytical Standard
97. Divk1c_000281
98. Spectrum1500801
99. Allantoin-[13c2,15n4]
100. Spbio_000237
101. Spbio_001924
102. Bpbio1_000005
103. Chembl593429
104. Allantoin [ep Monograph]
105. Dtxsid3020043
106. Sd 101 [who-dd]
107. 5-ureidohydantoin;glyoxyldiureide
108. Hms500o03
109. Kbio1_000281
110. Kbio2_001558
111. Kbio2_004126
112. Kbio2_006694
113. Kbio3_002051
114. Sd 101
115. Allantoin [usp Monograph]
116. Allantoin, >=98.0% (n)
117. Ninds_000281
118. Urea,5-dioxo-4-imidazolidinyl)-
119. Hms1568a05
120. Hms1921i10
121. Hms2092k16
122. Hms2095a05
123. Hms2268n08
124. Hms3712a05
125. Hms3885m08
126. Pharmakon1600-01500801
127. Amy13912
128. Bcp31832
129. Component Of Skin-balm (salt/mix)
130. Hy-n0543
131. 2,5-imidazolidinedione, 4-ureido-
132. Tox21_110395
133. Tox21_202087
134. Tox21_302912
135. Bbl027508
136. Ccg-39781
137. Nsc757792
138. S3856
139. Stl373778
140. Akos000120642
141. Akos016038547
142. Tox21_110395_1
143. Cs-7741
144. Db11100
145. Nsc-757792
146. Sdccgmls-0066595.p001
147. 1-(2,5-dioxoimidazolidin-4-yl);urea
148. Idi1_000281
149. Allantoin, P.a., 98.5-101.0%
150. N-(2,5-dioxo-4-imidazolidinyl)urea #
151. Ncgc00094854-01
152. Ncgc00094854-02
153. Ncgc00094854-03
154. Ncgc00094854-04
155. Ncgc00094854-05
156. Ncgc00094854-06
157. Ncgc00094854-07
158. Ncgc00256403-01
159. Ncgc00259636-01
160. Ac-11040
161. As-13865
162. Nci60_041675
163. Sodium Methanethiolate (~20% In Water)
164. N-(2,5-dioxo-4(1h)-imidazolidinyl)urea
165. Sbi-0051759.p002
166. A0211
167. Ab00052307
168. Ft-0604592
169. C01551
170. D85069
171. Urea, (2,5-dioxo-4-imidazolidinyl)- (9ci)
172. Ab00052307_11
173. 3-hydroxy-2-propyl-4-pentenoic Acid Ethyl Ester
174. Q409804
175. J-522839
176. Sr-01000766252-2
177. Sr-01000766252-3
178. Sr-01000766252-4
179. W-100104
180. Allantoin, European Pharmacopoeia (ep) Reference Standard
181. A999f0d6-0285-41d9-a6ba-b705987b663c
182. Allantoin, United States Pharmacopeia (usp) Reference Standard
183. Allantoin, Pharmaceutical Secondary Standard; Certified Reference Material
184. 5-ureidohydantoin; Glyoxyldiureide; Glyoxylic Diureide; Cordianine; Glyoxyldiureid; (2,5-dioxo-4-imidazolidinyl)urea
| Molecular Weight | 158.12 g/mol |
|---|---|
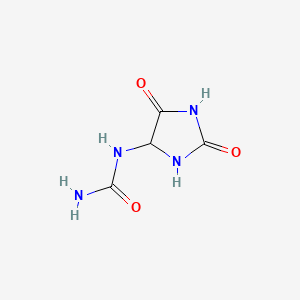
| Molecular Formula | C4H6N4O3 |
| XLogP3 | -2.2 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 1 |
| Exact Mass | 158.04399007 g/mol |
| Monoisotopic Mass | 158.04399007 g/mol |
| Topological Polar Surface Area | 113 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 225 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
A urea hydantoin that is found in URINE and PLANTS and is used in dermatological preparations.
National Library of Medicine, SIS; ChemIDplus Record for Allantoin ( 97-59-6), MESH Heading. Available from, as of February 1, 2006: https://chem.sis.nlm.nih.gov/chemidplus/chemidlite.jsp
Allantoin, a component in Comfrey, stimulates tissue repair and wound healing through cell proliferation. Allantoin has also had significant effect on cellular multiplication in degenerating and regenerating peripheral nerves.
PDR for Herbal Medicines; Medical Economics Co., Montvale, NJ. p. 212 (2000)
In humans, the allantoin to uric acid ratio in plasma increases during oxidative stress, thus this ratio has been suggested to be an in vivo marker for oxidative stress in humans.
PMID:16705445 Tsahar E et al; J Comp Physiol (B) 176 (7): 653-61 (2006)
Diagnostic marker for oxidative stress during antituberculous (anti-TB) therapy.
PMID:7546339 Walubo A et al; Biomed Environ Sci 8 (2): 106-13 (1995)
For more Therapeutic Uses (Complete) data for ALLANTOIN (8 total), please visit the HSDB record page.
Skin: For external use only. Ocular: Avoid contact with eyes. Sensitization: Mederma is contraindicated in individuals who have shown hypersensitivity to any of its components /Mederma/
Merz Pharmaceuticals, LLC; Material Safety Data Sheet, Mederma. December 12, 2002
Allantoin is commonly applied in a variety of topical vehicles or applications such as cosmetic creams, toothpastes, mouthwashes, shampoos, lipsticks, anti-acne products, and lotions for the purpose of moisturizing skin, enhancing the smoothness of skin, stimulating the healing of wounds, and soothing irritated skin.
FDA Label
Treatment of epidermolysis bullosa
There is no well controlled and appropriate data that can formally substantiate the pharmacodynamic properties of allantoin. Nevertheless, ongoing studies suggest that allantoin possesses moisturizing and keratolytic effects, as well as abilities to increase the water content of the extracellular matrix and enhance the desquamation of upper layers of dead skin cells, all of which are activities that can promote cell proliferation and facilitate wound healing.
Dermatologic Agents
Drugs used to treat or prevent skin disorders or for the routine care of skin. (See all compounds classified as Dermatologic Agents.)
Absorption
In studies on human subjects, a recovery of 19% and 34% of allantoin in the urine was observed but only in two individuals and only after the administration of massive doses of allantoin. After intravenous administration, recovery in the urine was practically quantitative with doses of 75 to 600 mgm in the human model. After 240 mgm, excretion continued for 72 hours in human subjects and the results were similar in regards to subcutaneous injection.
Route of Elimination
Urinary clearance is the predominant excretion route.
Clearance
Some studies suggest that the average renal clearance of allantoin in normal, healthy human subjects is approximately 123 cc per minute. It is generally agreed upon that exogenously administered allantoin is rapidly excreted.
Allantoin administered to dogs orally as solid or solution was excreted in the urine to an extent of between 35 and 92 per cent within 24 hours. No allantoin was recovered either in urine or feces when given to rabbits orally. In man the recovery was 19 and 34 per cent in two individuals after massive doses. After intravenous administration recovery in the urine was practically quantitative with doses of 75 to 600 mgm. in the dog and in man. After 240 mgm. in man excretion continued for 72 hours. The results were similar after subcutaneous injection. Uric acid injected intravenously into a dog was converted into allantoin within two hours.
Young EG et al; J Pharmacol Exptl Ther 81 (1): 1-9 (1944)
Uricase is the enzyme that possesses the functionality to convert uric acid to allantoin. Considering humans do not possess any endogenous uricase, uric acid is the only final breakdown product in the purine degradation of unwanted waste product purine nucleotides. The presence of allantoin in human urine is subsequently the result of non-enzymatic processes on uric acid with reactive oxygen species. Such non-enzymatic processes are consequently potentially suitable biomarkers for measuring oxidative stress in chronic illnesses and aging. Furthermore, as allantoin is found endogenously and is part of basic, natural metabolic pathways, no accumulation is expected of it. Additionally, allantoin is not believed to be metabolized to a measurable extent in humans and animals.
In humans, uric acid is the final breakdown product of unwanted purine nucleotides. Uric acid is the last stage in purine degradation, because humans lack the enzyme uricase which converts uric acid into allantoin.
PMID:15493112 Hediger MA; Ther Umsch 61 (9): 541-5 (2004)
Allantoin in the presence of calcium ions has been implicated as a potential toxic agent in Reye's syndrome. An investigation of possible alternative sources of allantoin in humans, which lack the enzyme uricase, has been initiated. Urate is a strong reducing agent which can reduce cytochrome c nonenzymatically, with the concomitant production of CO2 and H+. The stoichiometries measured for the various reactants and products were 1 urate:2 cytochrome c:1 H+:1 CO2. The initial reaction rate depended on the concentrations of both urate and cytochrome c, with reaction kinetics that were first order with respect to urate and second order with respect to cytochrome c. The participation of molecular oxygen in this reaction could not be detected. The pH and ionic strength optima for this reaction were determined to be 9.5-10.5 and 10(-5) M, respectively. Based on the results reported here, the following balanced equation can be written: urate-2 + 2 cytochrome c+3 + 2 H2O----allantoin + 2 cytochrome c+2 + H+ + HCO3-. /The authors/ propose that allantoin can be generated from the oxidation of urate by cytochrome c+3, and that this is a potential source of allantoin in human tissues.
PMID:3028263 Martens ME et al; Arch Biochem Biophys 252 (1):91-6 (1987)
Uric acid is the main nitrogenous waste product in birds but it is also known to be a potent antioxidant. Hominoid primates and birds lack the enzyme urate oxidase, which oxidizes uric acid to allantoin. Consequently, the presence of allantoin in their plasma results from non-enzymatic oxidation.
PMID:16705445 Tsahar E et al; J Comp Physiol (B) 176 (7): 653-61 (2006)
In most mammals purine degradation ultimately leads to the formation of allantoin. Humans lack the enzyme uricase, which catalyzes the conversion of uric acid to allantoin.
PMID:16375732 Masseoud D et al; Curr Pharm Des 11 (32): 4117-24 (2005)
For more Metabolism/Metabolites (Complete) data for ALLANTOIN (11 total), please visit the HSDB record page.
When studied in cattle, sheep, and horses, the half-life of allantoin is in the range of 1 to 2.5 hours.
There is no well controlled data that can formally substantiate the method of action. However, ongoing studies suggest that there may exist a histological wound healing profile induced by allantoin in rats that leads to the amelioration and fastening of the reestablishment of normal skin. This facilitation of wound healing is supported by observations that wounds inflicted to rat subjects to which topical allantoin preparations were applied histologically demonstrated increased vasodilation, presence of inflammatory exudates, number of inflammatory cells, angiogenesis, fibroblast proliferation, and increased collagen deposition when compared to rat subjects with wounds that did not receive any allantoin administration.
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
39
PharmaCompass offers a list of Glyoxyldiureide API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Glyoxyldiureide manufacturer or Glyoxyldiureide supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Glyoxyldiureide manufacturer or Glyoxyldiureide supplier.
PharmaCompass also assists you with knowing the Glyoxyldiureide API Price utilized in the formulation of products. Glyoxyldiureide API Price is not always fixed or binding as the Glyoxyldiureide Price is obtained through a variety of data sources. The Glyoxyldiureide Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Glyoxyldiureide manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Glyoxyldiureide, including repackagers and relabelers. The FDA regulates Glyoxyldiureide manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Glyoxyldiureide API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Glyoxyldiureide manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Glyoxyldiureide supplier is an individual or a company that provides Glyoxyldiureide active pharmaceutical ingredient (API) or Glyoxyldiureide finished formulations upon request. The Glyoxyldiureide suppliers may include Glyoxyldiureide API manufacturers, exporters, distributors and traders.
click here to find a list of Glyoxyldiureide suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Glyoxyldiureide DMF (Drug Master File) is a document detailing the whole manufacturing process of Glyoxyldiureide active pharmaceutical ingredient (API) in detail. Different forms of Glyoxyldiureide DMFs exist exist since differing nations have different regulations, such as Glyoxyldiureide USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Glyoxyldiureide DMF submitted to regulatory agencies in the US is known as a USDMF. Glyoxyldiureide USDMF includes data on Glyoxyldiureide's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Glyoxyldiureide USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Glyoxyldiureide suppliers with USDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Glyoxyldiureide as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Glyoxyldiureide API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Glyoxyldiureide as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Glyoxyldiureide and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Glyoxyldiureide NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Glyoxyldiureide suppliers with NDC on PharmaCompass.
Glyoxyldiureide Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Glyoxyldiureide GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Glyoxyldiureide GMP manufacturer or Glyoxyldiureide GMP API supplier for your needs.
A Glyoxyldiureide CoA (Certificate of Analysis) is a formal document that attests to Glyoxyldiureide's compliance with Glyoxyldiureide specifications and serves as a tool for batch-level quality control.
Glyoxyldiureide CoA mostly includes findings from lab analyses of a specific batch. For each Glyoxyldiureide CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Glyoxyldiureide may be tested according to a variety of international standards, such as European Pharmacopoeia (Glyoxyldiureide EP), Glyoxyldiureide JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Glyoxyldiureide USP).
