
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
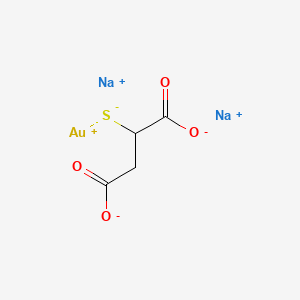

1. Aurolate
2. Aurothiomalate
3. Aurothiomalate, Sodium
4. Gold Disodium Thiomalate, Monohydrate
5. Gold Sodium Thiomalate
6. Gold Thiomalate
7. Gold Thiomalate, Sodium
8. Gold Thiomalic Acid
9. Mercaptobutanedioic Acid Monogold(1+) Sodium Salt
10. Miocrin
11. Miocrisin
12. Monogold (1+) Disodium Thiomalate
13. Myochrysine
14. Myocrisin
15. Myocrysine
16. Sodium Gold Thiomalate
17. Sodium Thiomalate, Gold
18. Sodium Thiomalatoaurate
19. Tauredon
20. Thiomalate, Gold
21. Thiomalatoaurate, Sodium
1. Tauredon
2. Gold Sodium Thiomalate
3. 12244-57-4
4. Myocrisin
5. Chrysothios
6. Butanedioic Acid, Mercapto-, Monogold(1+) Sodium Salt
7. Miochrysin
8. Myocrisine
9. Kidon
10. Taure(o)don
11. Disodium Aurothiomalate
12. Aurothiomala-natrium
13. Aurothiomalate Sodium
14. Aurothiomalato Sodico
15. Aurotiomalato Sodico
16. Natrii Aurothiomalas
17. Dinatrium 2-(aurothio)succinat
18. Hsdb 7173
19. Natrii Aurothiomalas [inn-latin]
20. Aurothiomalate De Sodium
21. Aurotiomalato Sodico [inn-spanish]
22. Disodium;gold(1+);2-sulfidobutanedioate
23. Einecs 235-479-7
24. Aurothiomalate De Sodium [inn-french]
25. Unii-e4768zy6gm
26. Succinic Acid, Mercapto-, Gold Sodium Salt
27. Disodium [2-(sulfanyl-kappas)butanedioato(3-)]aurate(2-)
28. Gold Sodium Thiomalate [usan:usp]
29. Na2[au(thiomalate)]
30. Sodium Aurothiomalate(i)
31. Sodium Aurum(i) Thiomalate
32. Schembl8548
33. Sodium Aurothiomalate [inn]
34. Disodium Thiomalato-s-gold(i)
35. E4768zy6gm
36. Disodium Thiomalato-s-aurate(i)
37. Chebi:35864
38. Mfcd00064304
39. Db09276
40. Sodium (1,2-dicarboxylatoethylthio)gold
41. [(1,2-dicarboxyethyl)thio]gold Disodium Salt
42. Mercaptobutanedioic Acid Gold(i) Disodium Salt
| Molecular Weight | 390.08 g/mol |
|---|---|
| Molecular Formula | C4H3AuNa2O4S |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 1 |
| Exact Mass | 389.921313 g/mol |
| Monoisotopic Mass | 389.921313 g/mol |
| Topological Polar Surface Area | 81.3 Ų |
| Heavy Atom Count | 12 |
| Formal Charge | 0 |
| Complexity | 126 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 4 |
... Gold sodium thiomalate ... /is/ indicated in the treatment of adult or juvenile rheumatoid arthritis. ... /This agent is/ usually used for treating patients who show evidence of continued or additional disease activity despite conservative therapy, e.g., with salicylates (especially aspirin) or other nonsteroidal anti-inflammatory agents, glucocorticoids, etc. /Included in US product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1586
Gold compounds are used in the treatment of these rheumatic conditions / psoriatic arthritis, Felty's syndrome/. /Gold compounds; NOT included in US product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1587
Patients with an impaired sulfoxidation ability (decreased ability to oxidize sulfhydryl-containing compounds) may be predisposed to ... /gold sodium thiomalate/ toxicity.
Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 1557
Vasomotor rections follow injections within minutes to hours and consist of weakness, dizziness, nausea, sweating and flushing, frequently accompanied by hypotension. Slower onset reactions consist of an exacerbation of joint pain and swelling, fatigue, and malaise that usually occur 6-24 hr after injection.
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. V2 160
Dermatitis is the most common reaction. Any eruption, especially if pruritic, that develops with treatment with myochrysine should be considered a reaction to gold until proven otherwise. Pruritus often exists before dermatitis becomes apparent, and there fore should be considered a warning signal of impending cutaneous reaction. The most serious form of cutaneous reaction is generalized exfoliative dermatitis which may lead to alopecia and shedding of nails. Gold dermatitis may be aggravated by exposure to sunlight or an actinic rash may develop.
Medical Economics Co; Physicians Desk Reference 50th ed p.1711 (1996)
Stomatitis is the second most common adverse reaction. Shallow ulcers on the buccal membranes, on the borders of the tongue and on the palate, or on the pharynx may occur as the only adverse reaction, or along with dermatitis. Sometimes diffuse glossitis or gingivitis develops. A metallic taste may precede these oral mucous membrane reactions and should be considered a warning signal.
Medical Economics Co; Physicians Desk Reference 50th ed p.1711 (1996)
For more Drug Warnings (Complete) data for GOLD SODIUM THIOMALATE (11 total), please visit the HSDB record page.
A disease-modifying antirheumatic drug (DMARD) indicated for the symptomatic treatment of arthritis.
Unknown, may decrease prostaglandin synthesis or may alter cellular mechanisms by inhibiting sulfhydryl systems.
Antirheumatic Agents
Drugs that are used to treat RHEUMATOID ARTHRITIS. (See all compounds classified as Antirheumatic Agents.)
M - Musculo-skeletal system
M01 - Antiinflammatory and antirheumatic products
M01C - Specific antirheumatic agents
M01CB - Gold preparations
M01CB01 - Sodium aurothiomalate
Absorption
Gold sodium thiomalate solutions are rapidly absorbed following IM injection, with peak serum concentrations occurring in 3-6 hours.
Route of Elimination
The major route of elimination of an IV dose of gold sodium thiomalate is urinary excretion, with a mean of 35% of the dose found in the urine in ten days. Fecal elimination accounts for an additional 9.4% of the IV dose excreted in ten days, probably as a result of biliary secretion.
Volume of Distribution
The apparent volume of distribution is 0.26 +/- 0.051 kg-1
Clearance
7.0 ml/ kg/day
Higher tissue levels occur with parenteral gold salts, with a mean steady state plasma level of 1 to 5 ug/ml. Drug is distributed widely throughout the body in lymph nodes, bone marrow, kidneys, liver, spleen, and tissues. About 85% to 90% is protein-bound.
Olson, K.R. (ed.) Poisoning & Drug Overdose. 3rd edition. Lange Medical Books/McGraw-Hill, New York, NY. 1999., p. 621
Gold has been shown to cross the placenta in pregnant women receiving gold sodium thiomalate. Small amounts of gold have been shown to be distributed into milk in women receiving ... gold sodium thiomalate.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 2848
Gold sodium thiomalate solutions are rapidly absorbed following IM injection, with peak serum concentrations occurring in 3-6 hours.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 2848
Following single 10-mg doses of gold sodium thiomalate, serum gold concentrations showed a biphasic decline with a relatively rapid early phase (serum half-life about 43 hours) and a slow late phase (serum half-life about 6 days). The slow phase of decline may result from excretion and the rapid phase of decline may result from tissue distribution. The true potential of gold compounds, including ... gold sodium thiomalate, to cumulate has not been clearly defined, but it is clear that substantially larger amounts of gold are retained in the body during therapy with parenteral gold compounds than during therapy with auranofin.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 2848
For more Absorption, Distribution and Excretion (Complete) data for GOLD SODIUM THIOMALATE (8 total), please visit the HSDB record page.
No data available.
For a patient receiving gold sodium thiomalate the principal gold species in the urine is [Au(CN)2]-, which is also seen in a low molecular weight infiltrate of the blood
PMID:8474063 Elder R et al; J Rheumatol. 20 (2): 268-72 (1993)
12.5 days
Following single 10-mg doses of gold sodium thiomalate, serum gold concentrations showed a biphasic decline with a relatively rapid early phase (serum half-life about 43 hours) and a slow late phase (serum half-life about 6 days).
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 2848
...Terminal log-linear phases corresponded to a mean disposition half-life of 25 days...
PMID:6430362 Massarella J et al; Biopharm Drug Dispos 5 (2): 101-7 (1984)
... the mean alpha half-lives were 0.738 and 1.78 hr for the iv and im routes, respectively. The corresponding terminal (beta) half-lives were 54.1 and 63.0 hr...
PMID:3150043 Melethil S et al; Pharm Res 4(4): 332-6 (1987)
Four normal male volunteers participated in a study designed to examine the disposition of gold given intramuscularly as gold sodium thiomalate. Blood samples were collected for 32 days following the administration of 10 mg of gold sodium thiomalate. .... Terminal log-linear phases corresponded to a mean disposition half-life of 25 days. ...
PMID:6430362 Massarella J et al; Biopharm Drug Dispos 5 (2): 101-7 (1984)
The precise mechanism of action is unknown. It is known that sodium aurothiomalate inhibits the synthesis of prostaglandins. The predominant action appears to be a suppressive effect on the synovitis of active rheumatoid disease.
...The effects of aurothiomalate, on basal and forskolin-activated adenylyl cyclase activity in human total lymphocyte membranes and in membranes of T and B lymphocyte subsets /was studied/. The gold compounds inhibited adenylyl cyclase activity. This inhibitory effect required the presence of both the sulfhydryl ligands and aurous cation. Regulation of lymphocyte adenylyl cyclase by gold compounds represents a potential mode of action of these drugs in rheumatic disease.
PMID:1642653 Lazarevic M et al; Arthritis Rheum 35 (8): 857-64 (1992)
Transcription factor NF-kappaB controls the expression of a number of genes including those for cell adhesion molecules such as E-selectin, ICAM- 1 and VCAM- 1. These cell adhesion molecules are known to play important roles in a critical step of tumor metastasis; the arrest of tumor cells on the venous or capillary bed of the target organ. NF-kappaB is activated by extracellular signals such as those elicited by the proinflammatory cytokines, TNF and IL-1. ...The adhesion of tumor cells to IL-1 beta-treated HUVEC /human umbilical vein endothelial cells/ was inhibited by gold compounds such as aurothiomalate.
Tozawa K et al; Cancer Letters 196 (1): 93-100
Gold dermatosis is mediated, at least in part, by allergic mechanisms
PMID:10583116 Rasanen L, et al; Br J Dermatol 141 (4): 683-8 (1999)

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
Related Excipient Companies

Excipients by Applications
ABOUT THIS PAGE
59
PharmaCompass offers a list of Gold Sodium Thiomalate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Gold Sodium Thiomalate manufacturer or Gold Sodium Thiomalate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Gold Sodium Thiomalate manufacturer or Gold Sodium Thiomalate supplier.
PharmaCompass also assists you with knowing the Gold Sodium Thiomalate API Price utilized in the formulation of products. Gold Sodium Thiomalate API Price is not always fixed or binding as the Gold Sodium Thiomalate Price is obtained through a variety of data sources. The Gold Sodium Thiomalate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Gold Sodium Thiomalate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Gold Sodium Thiomalate, including repackagers and relabelers. The FDA regulates Gold Sodium Thiomalate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Gold Sodium Thiomalate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Gold Sodium Thiomalate manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Gold Sodium Thiomalate supplier is an individual or a company that provides Gold Sodium Thiomalate active pharmaceutical ingredient (API) or Gold Sodium Thiomalate finished formulations upon request. The Gold Sodium Thiomalate suppliers may include Gold Sodium Thiomalate API manufacturers, exporters, distributors and traders.
click here to find a list of Gold Sodium Thiomalate suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
Gold Sodium Thiomalate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Gold Sodium Thiomalate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Gold Sodium Thiomalate GMP manufacturer or Gold Sodium Thiomalate GMP API supplier for your needs.
A Gold Sodium Thiomalate CoA (Certificate of Analysis) is a formal document that attests to Gold Sodium Thiomalate's compliance with Gold Sodium Thiomalate specifications and serves as a tool for batch-level quality control.
Gold Sodium Thiomalate CoA mostly includes findings from lab analyses of a specific batch. For each Gold Sodium Thiomalate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Gold Sodium Thiomalate may be tested according to a variety of international standards, such as European Pharmacopoeia (Gold Sodium Thiomalate EP), Gold Sodium Thiomalate JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Gold Sodium Thiomalate USP).
