
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
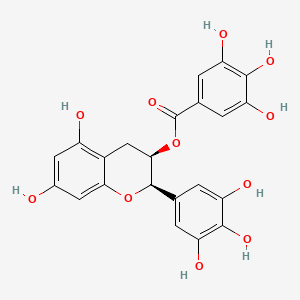

1. Egcg Cpd
2. Epigallo-catechin Gallate
3. Epigallocatechin Gallate
4. Epigallocatechin-3-gallate
5. Epigallocatechin-3-o-gallate
1. Egcg
2. 989-51-5
3. Epigallocatechin Gallate
4. Epigallocatechin 3-gallate
5. Tea Catechin
6. Epigallocatechin-3-gallate
7. (-)-epigallocatechin-3-o-gallate
8. Teavigo
9. Epigallocatechin-3-monogallate
10. (-)-epigallocatechin 3-gallate
11. (-)-epigallocatechol Gallate
12. Catechin Deriv.
13. Pf-egcg 90
14. Green Tea Extract
15. (2r,3r)-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)chroman-3-yl 3,4,5-trihydroxybenzoate
16. Nvp-xaa 723
17. Bqm438ctel
18. (2r,3r)-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)-3,4-dihydro-2h-chromen-3-yl 3,4,5-trihydroxybenzoate
19. [(2r,3r)-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)-3,4-dihydro-2h-chromen-3-yl] 3,4,5-trihydroxybenzoate
20. Chebi:4806
21. Chembl297453
22. [(2r,3r)-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)chroman-3-yl] 3,4,5-trihydroxybenzoate
23. Dsstox_cid_567
24. Dsstox_rid_78830
25. Dsstox_gsid_29889
26. (2r,3r)-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)-3,4-dihydro-2h-1-benzopyran-3-yl 3,4,5-trihydroxybenzoate
27. Benzoic Acid, 3,4,5-trihydroxy-, (2r,3r)-3,4-dihydro-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)-2h-1-benzopyran-3-yl Ester
28. Cas-989-51-5
29. Smr000449288
30. Ccris 3729
31. Sr-01000759328
32. Unii-bqm438ctel
33. L-epigallocatechin Gallate
34. Epigallocate
35. Epigalocatechin Gallate
36. (-)-egcg
37. Epigallocic Acid
38. Teatannin Ii
39. 2kdh
40. 3oob
41. 4awm
42. (-)-cis-2-(3,4,5-trihydroxyphenyl)-3,4-dihydro-1(2h)-benzopyran-3,5,7-triol 3-gallate
43. (-)-epigallocatechin 3-o-gallate
44. Kdh
45. Epigallocatechol, 3-gallate, (-)-
46. (-)-epigallocatechin Gallate (egcg)
47. Epigallocatcchin Gallate
48. Epigallocatechin-gallate
49. Epigallocatechol Gallate
50. Spectrum_000316
51. Specplus_000277
52. Spectrum2_000168
53. Spectrum3_000244
54. Spectrum4_001541
55. Spectrum5_000102
56. Galloyl-l-epigallocatechol
57. Egcg [who-dd]
58. Egcg [mi]
59. 3-o-galloylepigallocatechin
60. (-)-epigallocatechin Gallat
61. (-)-epigallocatehin Gallate
62. Schembl35258
63. Bspbio_001628
64. Epigallocatechin-gallate-(-)
65. Kbiogr_002002
66. Kbioss_000796
67. Spectrum210239
68. Cid_65064
69. Mls000758300
70. Mls001424000
71. Divk1c_006373
72. Spbio_000035
73. Epigallocatechin Monogallate, B
74. Gtpl7002
75. Megxp0_001166
76. Dtxsid1029889
77. Acon1_001054
78. Kbio1_001317
79. Kbio2_000796
80. Kbio2_003364
81. Kbio2_005932
82. Kbio3_001128
83. (-)-cis-3,3',4',5,5',7-hexahydroxy-flavane-3-gallate
84. Hms2051k21
85. Hms3649e08
86. 3-o-galloyl-(-)-epigallocatechin
87. Zinc3870412
88. Epigallocatechin 3-o-gallate
89. Tox21_201468
90. Tox21_303457
91. Bdbm50070942
92. Ccg-38378
93. Fr-109
94. Lmpk12030005
95. S2250
96. Akos015918182
97. Cs-1258
98. Db12116
99. Ds-9030
100. Epigallocatechin Gallate [inci]
101. Nc00078
102. Sdccgmls-0066550.p001
103. (-)-epigallocatechin Gallate, >=95%
104. (-)-epigallocatechin-3-gallate; Egcg
105. Ncgc00164319-01
106. Ncgc00164319-02
107. Ncgc00164319-03
108. Ncgc00164319-04
109. Ncgc00164319-06
110. Ncgc00257243-01
111. Ncgc00259019-01
112. (-)-epigallocatechin Gallate (85% (-)-epigallocatechin Gallate, 10% (-)-epigallocatechin, 5% (-)- Epicatechin Gallate)
113. Ac-34075
114. Bp-30205
115. Hy-13653
116. E0694
117. N1874
118. Sw197458-3
119. C09731
120. M01719
121. (-)-epigallocatechin Gallate, >=97.0% (hplc)
122. (-)-epigallocatechin Gallate, Analytical Standard
123. 989e515
124. A845931
125. Gallic Acid, 3-ester With Epigallocatechol, (-)-
126. Q393339
127. Sr-01000946601
128. (-)-epigallocatechin-3-o-gallate [usp-rs]
129. Q-100914
130. Sr-01000759328-5
131. Sr-01000759328-6
132. Sr-01000946601-1
133. Epigallocatechin-3-gallate 1000 Microg/ml In Acetonitrile
134. (-)-epigallocatechin Gallate, >=80% (hplc), From Green Tea
135. Epigallocatechin Gallate, Primary Pharmaceutical Reference Standard
136. (-)-epigallocatechin-3-o-gallate, United States Pharmacopeia (usp) Reference Standard
137. (2r,3r)-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)chroman-3-yl3,4,5-trihydroxybenzoate
138. [5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)chroman-3-yl] 2,3,4-trihydroxybenzoate
139. Epigallocatechin Gallate, Pharmaceutical Secondary Standard; Certified Reference Material
140. (-)-cis-3,4-dihydro-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)-1(2h)-benzopyran-3-yl Gallate
141. (-)-epigallocatechin Gallate (85% (-)-epigallocatechin Gallate, 10% (-)-epigallocatechin, 5% (-)-epicatechin Gallate)
142. (-)-epigallocatechin-3-o-gallate (egcg) (constituent Of Powdered Decaffeinated Green Tea Extract) [dsc]
143. (2r,3r)-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)-3,4-dihydro-2h-chromen-3-yl-3,4,5-trihydroxybenzoate
144. 3,4,5-trihydroxybenzoic Acid (2r,3r)-3,4-dihydro-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)-2h-1-benzopyran-3-yl Ester
145. 3,4-dihydro-5,7-dihydroxy-2r-(3,4,5-trihydroxyphenyl)-2h-1-benzopyran-3r-yl-3,4,5-trihydroxybenzoate
146. Benzoic Acid, 3,4,5-trihydroxy-, 3,4-dihydro-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)-2h-1-benzopyran-3-yl Ester, (2r-cis)-
147. Benzoic Acid, 3,4,5-trihydroxy-,(2r,3r)-3,4-dihydro-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)-2h-1-benzopyran-3-yl Ester
| Molecular Weight | 458.4 g/mol |
|---|---|
| Molecular Formula | C22H18O11 |
| XLogP3 | 1.2 |
| Hydrogen Bond Donor Count | 8 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 4 |
| Exact Mass | 458.08491139 g/mol |
| Monoisotopic Mass | 458.08491139 g/mol |
| Topological Polar Surface Area | 197 Ų |
| Heavy Atom Count | 33 |
| Formal Charge | 0 |
| Complexity | 667 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anticarcinogenic Agents
Agents that reduce the frequency or rate of spontaneous or induced tumors independently of the mechanism involved. (See all compounds classified as Anticarcinogenic Agents.)
Neuroprotective Agents
Drugs intended to prevent damage to the brain or spinal cord from ischemia, stroke, convulsions, or trauma. Some must be administered before the event, but others may be effective for some time after. They act by a variety of mechanisms, but often directly or indirectly minimize the damage produced by endogenous excitatory amino acids. (See all compounds classified as Neuroprotective Agents.)
Antioxidants
Naturally occurring or synthetic substances that inhibit or retard oxidation reactions. They counteract the damaging effects of oxidation in animal tissues. (See all compounds classified as Antioxidants.)
Antimutagenic Agents
Agents that reduce the frequency or rate of spontaneous or induced mutations independently of the mechanism involved. (See all compounds classified as Antimutagenic Agents.)
(-)-Epigallocatechin gallate has known human metabolites that include (-)-Epigallocatechin gallate, 3p-hydroxy-glucuronide and (-)-Epigallocatechin gallate, 4p-hydroxy-glucuronide.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
 Octavius has been empowering lives since 1980 by providing quality products like DC granules, APIs and FDFs.
Octavius has been empowering lives since 1980 by providing quality products like DC granules, APIs and FDFs.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 20492
Submission : 2007-04-27
Status : Active
Type : II









 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Market Place

REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
31
PharmaCompass offers a list of Green Tea Extract API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Green Tea Extract manufacturer or Green Tea Extract supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Green Tea Extract manufacturer or Green Tea Extract supplier.
PharmaCompass also assists you with knowing the Green Tea Extract API Price utilized in the formulation of products. Green Tea Extract API Price is not always fixed or binding as the Green Tea Extract Price is obtained through a variety of data sources. The Green Tea Extract Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Green Tea Extract manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Green Tea Extract, including repackagers and relabelers. The FDA regulates Green Tea Extract manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Green Tea Extract API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Green Tea Extract manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Green Tea Extract supplier is an individual or a company that provides Green Tea Extract active pharmaceutical ingredient (API) or Green Tea Extract finished formulations upon request. The Green Tea Extract suppliers may include Green Tea Extract API manufacturers, exporters, distributors and traders.
click here to find a list of Green Tea Extract suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Green Tea Extract DMF (Drug Master File) is a document detailing the whole manufacturing process of Green Tea Extract active pharmaceutical ingredient (API) in detail. Different forms of Green Tea Extract DMFs exist exist since differing nations have different regulations, such as Green Tea Extract USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Green Tea Extract DMF submitted to regulatory agencies in the US is known as a USDMF. Green Tea Extract USDMF includes data on Green Tea Extract's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Green Tea Extract USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Green Tea Extract suppliers with USDMF on PharmaCompass.
Green Tea Extract Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Green Tea Extract GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Green Tea Extract GMP manufacturer or Green Tea Extract GMP API supplier for your needs.
A Green Tea Extract CoA (Certificate of Analysis) is a formal document that attests to Green Tea Extract's compliance with Green Tea Extract specifications and serves as a tool for batch-level quality control.
Green Tea Extract CoA mostly includes findings from lab analyses of a specific batch. For each Green Tea Extract CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Green Tea Extract may be tested according to a variety of international standards, such as European Pharmacopoeia (Green Tea Extract EP), Green Tea Extract JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Green Tea Extract USP).
