
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
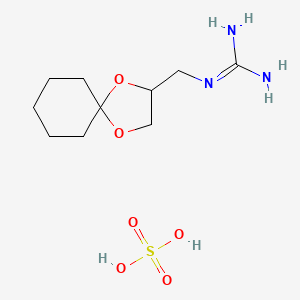

1. 22195-34-2
2. Anarel; Cl 1388r
3. 1-(1,4-dioxaspiro[4.5]decan-2-ylmethyl)guanidine Sulfate
4. Guanadrel Sulfate (200 Mg)
5. 2-(1,4-dioxaspiro[4.5]decan-3-ylmethyl)guanidine;sulfuric Acid
6. Guanadrel Hemisulfate
7. Schembl41051
8. Pharmakon1600-01505544
9. Ac8846
10. Mfcd08141811
11. Nsc760062
12. Ccg-213544
13. Sy250227
14. Sr-01000944213
15. Sr-01000944213-1
| Molecular Weight | 311.36 g/mol |
|---|---|
| Molecular Formula | C10H21N3O6S |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 2 |
| Exact Mass | 311.11510657 g/mol |
| Monoisotopic Mass | 311.11510657 g/mol |
| Topological Polar Surface Area | 166 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 326 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Antihypertensive Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Eleven patients with Graves' disease were treated with guanadrel sulfate and observed for changes in neuromuscular and cardiovascular manifestations. No notable changes in pulse rate or muscle strength were detected in either these patients during a 3-day pretreatment period or in 5 control patients with Graves' disease receiving placebo for 6 days. Thyroid hormone levels were not altered by 7 days of guanadrel sulfate therapy..., and no adverse side effects were encountered. Mean supine resting pulse fell from 102 +/- 6 (mean +/- SEM) to 90 +/- 3 beats/min (P<0.02). The patients' proximal and distal muscle strengths were initially decreased, when compared with healthy subjects, and improved substantially with guanadrel therapy. We conclude that guanadrel sulfate may be useful in the symptomatic management of patients with thyrotoxicosis.
PMID:580863 Rubenfeld S et al; Arch Intern Med 138 (7): 1106-8 (1978)
Because of the availability of a number of drugs that lower blood pressure without producing orthostatic hypotension, guanadrel is not employed in the monotherapy of hypertension, and is used chiefly as an additional agent in patients who have not achieved a satisfactory antihypertensive effect on two or more other agents.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 882
Patients and clinicians should be aware of the possibility of marked guanadrel induced orthostatic hypotension and its consequences of dizziness, weakness, and fainting. Clinicians should monitor both erect and supine blood pressures, as supine blood pressures alone may not reveal the possibility of orthostatic hypotension. Patients should be cautioned to avoid sudden or prolonged standing (especially in the morning) or exercise and should be advised of measures to take if dizziness or weakness occurs (eg, lying or sitting down). A hot environment, alcohol ingestion, or fever may aggravate postural hypotension. Because of the risk of orthostatic hypotension, the drug should be used cautiously in geriatric patients. Geriatric patients may be more sensitive to sympathetic inhibition than younger patients, because they frequently have impaired cardiovascular reflexes, making them more susceptible to hypotension.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 1829
Guanadrel should be used cautiously in patients with bronchial asthma, since asthma may be aggravated by catecholamine depletion in these patients and because sympathomimetic amines used in the treatment of asthma may interfere with the hypotensive effect of guanadrel. The drug also should be used with caution in patients with peptic ulcer disease, as this condition may be aggravated by a guanadrel induced relative increase in parasympathetic tone.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 1829
Guanadrel therapy should be discontinued 2-3 days prior to elective surgery to reduce the possibility of cardiovascular collapse and cardiac arrest during anesthesia. If emergency surgery is necessary, the anesthesiologist should be advised that the patient is receiving the drug and preanesthetic and anesthetic agents should be administered cautiously and in reduced dosage. Vasopressors should be administered with caution since guanadrel may enhance the pressor and arrhythmogenic responses to these drugs.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 1829
Guanadrel should be used with caution in patients who may be adversely affected by sodium and water retention; however, concomitant use of a diuretic will usually overcome this effect.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 1829
For more Drug Warnings (Complete) data for GUANADREL SULFATE (19 total), please visit the HSDB record page.
Guanadrel sulfate is rapidly and almost completely absorbed following oral administration. Peak plasma concentrations usually are achieved 1.5-2 hr after oral administration. The hypotensive effect of guanadrel sulfate usually has an onset of 0.5-2 hrs, peaks at 4-6 hr, and persists for 4-14 hr.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 1830
Approximately 20% of guanadrel is bound to plasma proteins over a wide concentration range. The drug is widely distributed into most body tissues and fluids. Little, if any, of the drug crosses the blood-brain barrier or distributes into the eye. It is not known whether guanadrel is distributed into milk or crosses the placenta in humans. The drug has been shown to cross the placenta in small concentrations in mice
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 1830
Guanadrel is cleared from the body by both renal and nonrenal disposition. Its elimination is impaired in patients with renal insufficiency; total-body clearance was reduced by 4- to 5-fold in a group of patients with a clearance of creatinine averaging 13 ml per minute.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 882
Approximately 40-50% of the drug is metabolized in the liver ... . Guanadrel and its metabolites are excreted principally in urine. Approximately 85% of an oral dose of the drug is excreted in urine within 24 hrs; 40-50% of the dose is excreted in urine unchanged.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 1830
Approximately 40-50% of the drug is metabolized in the liver to 2,3-dihydroxypropylguanidine and several unidentified metabolites. The hypotensive activity of the metabolites is not known.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 1830
Plasma concentrations of guanadrel appear to decline in a biphasic manner. There is considerable interindividual variation in plasma half-life of the drug. In patients with normal renal function, guanadrel has a plasma half-life in the initial phase of about 2 hr (range: 1-4) and an elimination half-life of about 10-12 hr (range: 5-45).
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 1830
Guanadrel is targeted uniquely to the peripheral adrenergic neuron, where it inhibits sympathetic function. The drug reaches its site of action by active transport into the neuron by the same transporter that is responsible for the reuptake of norephinephrine. In the neuron, guanadrel is concentrated within the neurosecretory vesicles, where it replaces norepinephrine. During chronic administration, guanadrel acts as a "substitute neurotransmitter," in that it is present in storage vesicles, it depletes the normal transmitter, and it can be released by stimuli that normally release norepinephrine. This replacement of norephinephrine with an inactive transmitter is probably the principal mechanism of its neuron-blocking action.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 881
Guanadrel sulfate, like guanethidine, produces a selective block of efferent, peripheral sympathetic pathways. The drug depletes norepinephrine stores from adrenergic nerve endings and, unlike guanethidine, from the adrenal medulla; guanadrel also prevents the release of norepinephrine from adrenergic nerve endings in response to sympathetic nerve stimulation. Guanadrel reportedly depletes norepinephrine stores in the GI tract to a lesser extent than does guanethidine. Chronic administration of guanadrel results in an increased sensitivity of effector cells to catecholamines. Following oral administration of guanadrel, depletion of catecholamine stores produces a fall in blood pressure which usually, but not always, is accompanied by a 5 to 10 beat/min reduction in heart rate. Venous dilation and peripheral pooling of blood may cause a slight decrease or no change in cardiac output. Total peripheral resistance usually is decreased slightly.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 1830
ABOUT THIS PAGE
70
PharmaCompass offers a list of Guanadrel API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Guanadrel manufacturer or Guanadrel supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Guanadrel manufacturer or Guanadrel supplier.
PharmaCompass also assists you with knowing the Guanadrel API Price utilized in the formulation of products. Guanadrel API Price is not always fixed or binding as the Guanadrel Price is obtained through a variety of data sources. The Guanadrel Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Guanadrel manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Guanadrel, including repackagers and relabelers. The FDA regulates Guanadrel manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Guanadrel API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Guanadrel supplier is an individual or a company that provides Guanadrel active pharmaceutical ingredient (API) or Guanadrel finished formulations upon request. The Guanadrel suppliers may include Guanadrel API manufacturers, exporters, distributors and traders.
click here to find a list of Guanadrel suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Guanadrel DMF (Drug Master File) is a document detailing the whole manufacturing process of Guanadrel active pharmaceutical ingredient (API) in detail. Different forms of Guanadrel DMFs exist exist since differing nations have different regulations, such as Guanadrel USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Guanadrel DMF submitted to regulatory agencies in the US is known as a USDMF. Guanadrel USDMF includes data on Guanadrel's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Guanadrel USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Guanadrel suppliers with USDMF on PharmaCompass.
Guanadrel Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Guanadrel GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Guanadrel GMP manufacturer or Guanadrel GMP API supplier for your needs.
A Guanadrel CoA (Certificate of Analysis) is a formal document that attests to Guanadrel's compliance with Guanadrel specifications and serves as a tool for batch-level quality control.
Guanadrel CoA mostly includes findings from lab analyses of a specific batch. For each Guanadrel CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Guanadrel may be tested according to a variety of international standards, such as European Pharmacopoeia (Guanadrel EP), Guanadrel JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Guanadrel USP).

