

Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
API
0
FDF
0
FDA Orange Book

0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
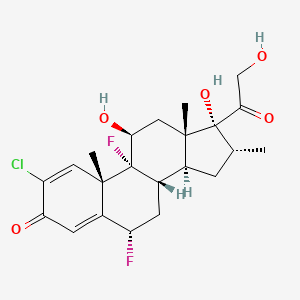

1. 2-chloro-6alpha, 9-difluoro-11beta, 17,21-trihydroxy-16alpha-methylpregna-1,4-dien-3,20-dione
2. C 48,401-ba
3. C-48,401-ba
4. Halometasone Monohydrate
5. Sicorten
1. 50629-82-8
2. 2-chloroflumethasone
3. Halometasone [inn]
4. Halometasone Monohydrate
5. Halometasone Hydrate
6. J69z9uu41z
7. Halometasone (inn)
8. Pregna-1,4-diene-3,20-dione, 2-chloro-6,9-difluoro-11,17,21-trihydroxy-16-methyl-, (6alpha,11beta,16alpha)-
9. Halometasona
10. Halometasonum
11. Smr000466330
12. Halometasonum [inn-latin]
13. Halometasona [inn-spanish]
14. Unii-j69z9uu41z
15. (6s,8s,9r,10s,11s,13s,14s,16r,17r)-2-chloro-6,9-difluoro-11,17-dihydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-3-one
16. Einecs 256-664-9
17. Halometasone [mi]
18. Schembl4872
19. Halometasone [mart.]
20. Mls000759439
21. Mls001424089
22. Halometasone [who-dd]
23. Chembl1587228
24. Dtxsid1048382
25. Chebi:135724
26. Hms2051c16
27. Hms2232n17
28. Zinc3938673
29. Akos025402048
30. Ac-3528
31. Ccg-100880
32. Db13728
33. Nc00130
34. 2-chlor-6alpha,9-difluor-11beta,17,21-trihydroxy-16alpha-methyl-1,4-pregnadien-3,20-dion
35. As-78199
36. Cs-0453061
37. D07202
38. Ab00639905-06
39. 629h828
40. Q-101370
41. Q10910139
42. (6s,8s,9r,10s,11s,13s,14s,16r,17r)-2-chloro-6,9-difluoro-11,17-dihydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3h-cyclopenta[a]phenanthren-3-one
43. 2-chloro-6.alpha.,9-difluoro-11.beta.,17,21-trihydroxy-16.alpha.-methylpregna-1,4-diene-3,20-dione
44. 2-chloro-6alpha,9-difluoro-11beta,17,21-trihydroxy-16alpha-methylpregna-1,4-diene-3,20-dione
| Molecular Weight | 444.9 g/mol |
|---|---|
| Molecular Formula | C22H27ClF2O5 |
| XLogP3 | 2.6 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 2 |
| Exact Mass | 444.1515080 g/mol |
| Monoisotopic Mass | 444.1515080 g/mol |
| Topological Polar Surface Area | 94.8 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 889 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 9 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Inflammatory Agents
Substances that reduce or suppress INFLAMMATION. (See all compounds classified as Anti-Inflammatory Agents.)
Dermatologic Agents
Drugs used to treat or prevent skin disorders or for the routine care of skin. (See all compounds classified as Dermatologic Agents.)
Glucocorticoids
A group of CORTICOSTEROIDS that affect carbohydrate metabolism (GLUCONEOGENESIS, liver glycogen deposition, elevation of BLOOD SUGAR), inhibit ADRENOCORTICOTROPIC HORMONE secretion, and possess pronounced anti-inflammatory activity. They also play a role in fat and protein metabolism, maintenance of arterial blood pressure, alteration of the connective tissue response to injury, reduction in the number of circulating lymphocytes, and functioning of the central nervous system. (See all compounds classified as Glucocorticoids.)
D - Dermatologicals
D07 - Corticosteroids, dermatological preparations
D07A - Corticosteroids, plain
D07AC - Corticosteroids, potent (group iii)
D07AC12 - Halometasone
Global Sales Information

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
