
Synopsis
Synopsis
0
VMF
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Haldol
1. 52-86-8
2. Haldol
3. Serenace
4. Aloperidin
5. Eukystol
6. Aloperidol
7. Serenelfi
8. Brotopon
9. Serenase
10. Dozic
11. Linton
12. Einalon S
13. Aloperidolo
14. Galoperidol
15. Halojust
16. Halopoidol
17. Ulcolind
18. Uliolind
19. Halopal
20. Keselan
21. Mixidol
22. Pernox
23. Sernas
24. Sernel
25. Aldo
26. Lealgin Compositum
27. Bioperidolo
28. Sigaperidol
29. Peluces
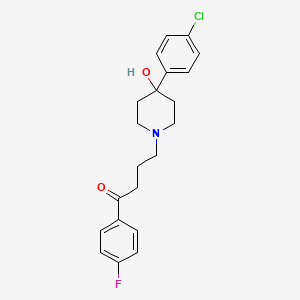
30. 4-[4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl]-1-(4-fluorophenyl)butan-1-one
31. Mcn-jr-1625
32. Haloperidolum
33. Aloperidon
34. 177716-59-5
35. R-1625
36. 4-(4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl)-1-(4-fluorophenyl)butan-1-one
37. Nsc 170973
38. R 1625
39. 1-(3-p-fluorobenzoylpropyl)-4-p-chlorophenyl-4-hydroxypiperidine
40. Haloperidol (haldol)
41. 4'-fluoro-4-(4-(p-chlorophenyl)-4-hydroxypiperidinyl)butyrophenone
42. 4'-fluoro-4-(4-hydroxy-4-(4'-chlorophenyl)piperidino)butyrophenone
43. 4-[4-(4-chlorophenyl)-4-hydroxy-1-piperidinyl]-1-(4-fluorophenyl)-1-butanone
44. Chembl54
45. Haldol (tn)
46. Haldol Solutab
47. Nsc-170973
48. Nsc-615296
49. 4-(4-hydroxy-4'-chloro-4-phenylpiperidino)-4'-fluorobutyrophenone
50. 1-butanone, 4-(4-(4-chlorophenyl)-4-hydroxy-1-piperidinyl)-1-(4-fluorophenyl)-
51. 4-(4-(4-chlorophenyl)-4-hydroxy-1-piperidinyl)-1-(4-fluorophenyl)-1-butanone
52. Mls000028450
53. Chebi:5613
54. Fortunan
55. Halosten
56. 4-(4-(p-chlorophenyl)-4-hydroxypiperidino)-4'-fluorobutyrophenone
57. 4-[4-(p-chlorophenyl)-4-hydroxypiperidino]-4'-fluorobutyrophenone
58. J6292f8l3d
59. Butyrophenone, 4'-fluoro-4-(4-(p-chlorophenyl)-4-hydroxypiperidino)-
60. 1-butanone, 4-[4-(4-chlorophenyl)-4-hydroxy-1-piperidinyl]-1-(4-fluorophenyl)-
61. Nsc170973
62. Nsc615296
63. Cas-52-86-8
64. Ncgc00015500-10
65. Aloperidolo [dcit]
66. Halidol
67. Halopidol
68. Pekuces
69. Smr000058303
70. Halol
71. Aloperidolo [italian]
72. Poly[2-methoxy-5-(3',7'-dimethyloctyloxy)-1,4-phenylenevinylene]
73. 4-(4-(para-chlorophenyl)-4-hydroxypiperidino)-4'-fluorobutyrophenone
74. Butyrophenone, 4-(4-(p-chlorophenyl)-4-hydroxypiperidino)-4'-fluoro-
75. Gamma-(4-(p-chlorophenyl)-4-hydroxypiperidino)-p-fluorobutyrophenone
76. 4-[4-(4-chlorophenyl)-4-hydroxy-1-piperidyl]-1-(4-fluorophenyl)-butan-1-one
77. Poly[[2-[(3,7-dimethyloctyl)oxy]-5-methoxy-1,4-phenylene]-1,2-ethenediyl]
78. Dsstox_cid_14150
79. Dsstox_rid_79117
80. Dsstox_gsid_34150
81. Haloperidolum [inn-latin]
82. Neurodol
83. Butyrophenone, 4-[4-(p-chlorophenyl)-4-hydroxypiperidino]-4'-fluoro-
84. Gamma-(4-(p-chlorophenyl)-4-hydroxpiperidino)-p-fluorbutyrophenone
85. Ccris 1630
86. Hsdb 3093
87. Sr-01000003076
88. Einecs 200-155-6
89. Nsc 615296
90. Brn 0331267
91. Duraperidol
92. Unii-j6292f8l3d
93. Epoxy Resins
94. 4'-fluoro-4-[4-hydroxy-4-(4'-chlorophenyl)piperidino]butyrophenone
95. Gamma-[4-(p-chlorophenyl)-4-hydroxypiperidino]-p-fluorobutyrophenone
96. 4-[4-(para-chlorophenyl)-4-hydroxypiperidino]-4'-fluorobutyrophenone
97. Picroside-iii
98. Haloperidol, 1
99. Haloperidol, Powder
100. Prestwick_250
101. N--boc-l-asparagine
102. Haloperidol [usan:usp:inn:ban:jan]
103. Spectrum_000861
104. Tocris-0931
105. Starbld0018801
106. Opera_id_446
107. 4'-fluoro-4-(4-hydroxy-4-p-chlorophenylpiperidino)butyrophenone
108. Haloperidol [mi]
109. 4-[4-(4-chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1-(4-fluoro-phenyl)-butan-1-one;propionate(hcl)
110. 4-[4-(4-chlorophenyl)-4-hydroxypiperidino]-4'-fluorobutyrophenone
111. Gamma-(4-(p-chlorophenyl)-4-hydroxypiperidino)-p-fluorbutyrophenone
112. Prestwick0_000115
113. Prestwick1_000115
114. Prestwick2_000115
115. Prestwick3_000115
116. Spectrum2_001268
117. Spectrum3_000448
118. Spectrum4_000570
119. Spectrum5_000788
120. Haloperidol [inn]
121. Haloperidol [jan]
122. Lopac-h-1512
123. Haloperidol [hsdb]
124. Haloperidol [usan]
125. Biomol-nt_000035
126. Gtpl86
127. Probes1_000255
128. Probes2_000296
129. H 1512
130. Haloperidol [vandf]
131. Schembl8264
132. Haloperidol [mart.]
133. Lopac0_000583
134. Oprea1_509923
135. Bspbio_000130
136. Bspbio_002096
137. Haloperidol [usp-rs]
138. Haloperidol [who-dd]
139. Haloperidol [who-ip]
140. Kbiogr_000980
141. Kbiogr_002390
142. Kbioss_001341
143. Kbioss_002395
144. 5-21-02-00377 (beilstein Handbook Reference)
145. Mls001146904
146. Bidd:gt0128
147. Divk1c_000654
148. Spectrum1500325
149. Haloperidol-[chlorophenyl-d4]
150. Spbio_001236
151. Spbio_002069
152. Mcm-jr-1625
153. Bpbio1_000144
154. Bpbio1_001231
155. Haloperidol (jp17/usp/inn)
156. Dtxsid4034150
157. Bdbm21398
158. Hms502a16
159. Kbio1_000654
160. Kbio2_001341
161. Kbio2_002390
162. Kbio2_003909
163. Kbio2_004958
164. Kbio2_006477
165. Kbio2_007526
166. Kbio3_001316
167. Kbio3_002869
168. Lnepoxffqsencj-uhfffaoysa-
169. Haloperidol [orange Book]
170. Cmap_000037
171. Haloperidol For System Suitability
172. Ninds_000654
173. Ac250
174. Haloperidol [ep Monograph]
175. Haloperidol [usp Impurity]
176. Haloperidol For Peak Identification
177. Hms1568g12
178. Hms1920d03
179. Hms2089m15
180. Hms2091j09
181. Hms2095g12
182. Hms2234p08
183. Hms3261f08
184. Hms3370h11
185. Hms3657i13
186. Hms3712g12
187. Pharmakon1600-01500325
188. Zinc537822
189. Haloperidol [usp Monograph]
190. Bcp33202
191. Cha71659
192. Haloperidol 1.0 Mg/ml In Methanol
193. Str04750
194. Tox21_110162
195. Tox21_300475
196. Tox21_500583
197. A0h334
198. Ccg-36042
199. Ccg-39111
200. Haloperidolum [who-ip Latin]
201. Nsc757054
202. Stl417208
203. Vesalium Component Haloperidol
204. 4-[4-(4-chlorophenyl)-4-hydroxy-1-piperidyl]-1-(4-fluorophenyl)butan-1-one
205. Akos000280660
206. Tox21_110162_1
207. At13670
208. Cs-1971
209. Db00502
210. Lp00583
211. Nsc-757054
212. Sdccgsbi-0050565.p005
213. Haloperidol Component Of Vesalium
214. Idi1_000654
215. Mrf-0000027
216. Qtl1_000042
217. Wln: T6ntj A3vr Df& Dq Dr Dg
218. Ncgc00015500-01
219. Ncgc00015500-02
220. Ncgc00015500-03
221. Ncgc00015500-04
222. Ncgc00015500-05
223. Ncgc00015500-06
224. Ncgc00015500-07
225. Ncgc00015500-08
226. Ncgc00015500-09
227. Ncgc00015500-11
228. Ncgc00015500-12
229. Ncgc00015500-13
230. Ncgc00015500-14
231. Ncgc00015500-15
232. Ncgc00015500-16
233. Ncgc00015500-17
234. Ncgc00015500-19
235. Ncgc00015500-24
236. Ncgc00015500-32
237. Ncgc00016234-01
238. Ncgc00023875-02
239. Ncgc00023875-04
240. Ncgc00023875-05
241. Ncgc00023875-06
242. Ncgc00023875-07
243. Ncgc00023875-08
244. Ncgc00023875-09
245. Ncgc00254503-01
246. Ncgc00261268-01
247. Ac-19691
248. Bh166165
249. Hy-14538
250. Sbi-0050565.p004
251. Haloperidol Decanoate Impurity, Haloperidol-
252. Sc 170973
253. Ab00052008
254. Eu-0100583
255. Ft-0669100
256. Ft-0669101
257. Ft-0697842
258. H0912
259. N1910
260. S1920
261. Sw196557-4
262. C01814
263. D00136
264. Vu0239704-10
265. Ab00052008-21
266. Ab00052008-22
267. Ab00052008_23
268. Ab00052008_24
269. A899749
270. L000288
271. Q251347
272. Sr-01000003076-2
273. Sr-01000003076-8
274. W-105791
275. Brd-k67783091-001-04-8
276. Brd-k67783091-001-05-5
277. Brd-k67783091-003-03-6
278. Haloperidol Decanoate Impurity G [ep Impurity]
279. Sr-01000003076-11
280. Haloperidol, European Pharmacopoeia (ep) Reference Standard
281. .gamma.-[4-(p-chlorphenyl)-4-hydroxpiperidino]-p-fluorbutyrophenone
282. Haloperidol Decanoate Impurity, Haloperidol- [usp Impurity]
283. Haloperidol, United States Pharmacopeia (usp) Reference Standard
284. .gamma.-(4-(p-chlorophenyl)-4-hydroxpiperidino)-p-fluorbutyrophenone
285. Haloperidol, Pharmaceutical Secondary Standard; Certified Reference Material
286. 4-[4-(4-chlorophenyl)-4-hydroxy-1-piperidinyl]-1-(4-fluorophenyl) -1-butanone
287. Haloperidol For Peak Identification, European Pharmacopoeia (ep) Reference Standard
288. Haloperidol For System Suitability, European Pharmacopoeia (ep) Reference Standard
289. Haloperidol Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
| Molecular Weight | 375.9 g/mol |
|---|---|
| Molecular Formula | C21H23ClFNO2 |
| XLogP3 | 3.2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 6 |
| Exact Mass | 375.1401348 g/mol |
| Monoisotopic Mass | 375.1401348 g/mol |
| Topological Polar Surface Area | 40.5 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 451 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Haldol |
| PubMed Health | Haloperidol Decanoate (Injection) |
| Drug Classes | Antipsychotic |
| Drug Label | Haloperidol is the first of the butyrophenone series of major antipsychotics. The chemical designation is 4-[4-(p-chlorophenyl)-4-hydroxypiperidino]-4'-fluorobutyrophenone and it has the following structural formula:HALDOL (haloperidol) is available. |
| Active Ingredient | Haloperidol decanoate; Haloperidol lactate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 50mg base/ml; eq 5mg base/ml; eq 100mg base/ml |
| Market Status | Prescription |
| Company | Janssen Pharms |
| 2 of 4 | |
|---|---|
| Drug Name | Haloperidol |
| PubMed Health | Haloperidol |
| Drug Classes | Antipsychotic |
| Drug Label | Haloperidol is the first of the butyrophenone series of major tranquilizers. The chemical designation is 4-[4-(p-chloro-phenyl)-4-hydroxypiperidino]-4fluorobutyrophenone and it has the following structural formula:C21H23ClFNO2 375.87Haloperidol... |
| Active Ingredient | Haloperidol; Haloperidol lactate |
| Dosage Form | Tablet; Concentrate; Injectable |
| Route | injection; oral; Injection; Oral |
| Strength | 0.5mg; 1mg; eq 2mg base/ml; eq 5mg base/ml; 5mg; 5mg in 10ml vials; 2mg; 10mg; 20mg |
| Market Status | Prescription |
| Company | Silarx; Gland Pharma; Pharm Assoc; Teva Pharms Usa; Sandoz; Teva Pharms; Fresenius Kabi Usa; Eurohlth Intl; Sagent Pharms; Zydus Pharms Usa; Agila Speclts; Mylan |
| 3 of 4 | |
|---|---|
| Drug Name | Haldol |
| PubMed Health | Haloperidol Decanoate (Injection) |
| Drug Classes | Antipsychotic |
| Drug Label | Haloperidol is the first of the butyrophenone series of major antipsychotics. The chemical designation is 4-[4-(p-chlorophenyl)-4-hydroxypiperidino]-4'-fluorobutyrophenone and it has the following structural formula:HALDOL (haloperidol) is available. |
| Active Ingredient | Haloperidol decanoate; Haloperidol lactate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 50mg base/ml; eq 5mg base/ml; eq 100mg base/ml |
| Market Status | Prescription |
| Company | Janssen Pharms |
| 4 of 4 | |
|---|---|
| Drug Name | Haloperidol |
| PubMed Health | Haloperidol |
| Drug Classes | Antipsychotic |
| Drug Label | Haloperidol is the first of the butyrophenone series of major tranquilizers. The chemical designation is 4-[4-(p-chloro-phenyl)-4-hydroxypiperidino]-4fluorobutyrophenone and it has the following structural formula:C21H23ClFNO2 375.87Haloperidol... |
| Active Ingredient | Haloperidol; Haloperidol lactate |
| Dosage Form | Tablet; Concentrate; Injectable |
| Route | injection; oral; Injection; Oral |
| Strength | 0.5mg; 1mg; eq 2mg base/ml; eq 5mg base/ml; 5mg; 5mg in 10ml vials; 2mg; 10mg; 20mg |
| Market Status | Prescription |
| Company | Silarx; Gland Pharma; Pharm Assoc; Teva Pharms Usa; Sandoz; Teva Pharms; Fresenius Kabi Usa; Eurohlth Intl; Sagent Pharms; Zydus Pharms Usa; Agila Speclts; Mylan |
Anti-Dyskinesia Agents; Antiemetics; Antipsychotic Agents, Butyrophenone; Dopamine Antagonists
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Haloperidol is indicated for the management of the manifestations of acute and chronic psychotic disorders including schizophrenia, manic states, and drug-induced psychoses, such as steroid psychosis. It may also be useful in the management of aggressive and agitated patients, including patients with organic mental syndrome or mental retardation. Haloperidol decanoate, a long-acting parenteral from, is intended for maintenance use in the management of patients requiring prolonged parenteral therapy, as in chronic schizophrenia. /Included in US product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1456
Haloperidol is effective in the treatment of children with severe behavior problems of apparently unprovoked, combative, explosive hyperexcitability. It is also effective in the short-term treatment of hyperactivity in children who show excessive motor activity with accompanying conduct disorders such as aggressiveness, impulsiveness, easy frustration, short attention span, and/or rapid mood fluctuations. In these two groups of children, haloperidol should be tried only in patients who fail to respond to psychotherapy or other non-neuroleptic medication. /Included in US product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1456
Haloperidol is used to control tics and vocalizations of Tourette's syndrome in children and adults. /Included in US product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1456
For more Therapeutic Uses (Complete) data for HALOPERIDOL (8 total), please visit the HSDB record page.
Pregnancy risk category: C /RISK CANNOT BE RULED OUT. Adequate, well controlled human studies are lacking, and animal studies have shown risk to the fetus or are lacking as well. There is a chance of fetal harm if the drug is given during pregnancy; but the potential benefits may outweigh the potential risk./
Physicians Desk Reference. 58th ed. Thomson PDR. Montvale, NJ 2004., p. 2444
Extrapyramidal reactions occur frequently with haloperidol, especially during the first few days of therapy. In most patients, these reactions consist of parkinsonian symptoms (e.g., marked drowsiness and lethargy, drooling or hypersalivation, fixed stare), which are mild to moderate in severity and are usually reversible following discontinuance of the drug. Other adverse neuromuscular reactions have been reported less frequently, but are often more severe, and include feelings of motor restlessness (i.e., akathisia), tardive dystonia, and dystonic reactions (e.g., hyperreflexia, opisthotonos, oculogyric crisis, torticollis, trismus).
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 2289
Possible drowsiness or dizziness; caution when driving, using machinery, or doing things requiring alertness. Possible dizziness or lightheadedness; caution when getting up suddenly from a lying or sitting position.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1459
Because of the possibility of transient hypotension and/or precipitation of angina, haloperidol should be used with caution in patients with severe cardiovascular disorders. If hypotension occurs, metaraminol, norepinephrine, or phenylephrine may be used; epinephrine should not be used since haloperidol causes a reversal of epinephrine's vasopressor effects and a further lowering of blood pressure.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 2290
For more Drug Warnings (Complete) data for HALOPERIDOL (24 total), please visit the HSDB record page.
Haloperidol is indicated for a number of conditions including for the treatment of schizophrenia, for the manifestations of psychotic disorders, for the control of tics and vocal utterances of Tourettes Disorder in children and adults, for treatment of severe behavior problems in children of combative, explosive hyperexcitability (which cannot be accounted for by immediate provocation). Haloperidol is also indicated in the short-term treatment of hyperactive children who show excessive motor activity with accompanying conduct disorders consisting of some or all of the following symptoms: impulsivity, difficulty sustaining attention, aggressivity, mood lability, and poor frustration tolerance. Haloperidol should be reserved for these two groups of children only after failure to respond to psychotherapy or medications other than antipsychotics.
FDA Label
Use of the first-generation antipsychotics (including haloperidol) is considered highly effective for the management of the "positive" symptoms of schizophrenia including hallucinations, hearing voices, aggression/hostility, disorganized speech, and psychomotor agitation. However, this class is limited by the development of movement disorders such as drug-induced parkinsonism, akathisia, dystonia, and tardive dyskinesia, and other side effects including sedation, weight gain, and prolactin changes. Compared to the lower-potency first-generation antipsychotics such as [DB00477], [DB01624], [DB00623], and [DB01403], haloperidol typically demonstrates the least amount of side effects within class, but demonstrates a stronger disposition for causing extrapyramidal symptoms (EPS). Lowpotency medications have a lower affinity for dopamine receptors so that a higher dose is required to effectively treat symptoms of schizophrenia. In addition, they block many receptors other than the primary target (dopamine receptors), such as cholinergic or histaminergic receptors, resulting in a higher incidence of side effects such as sedation, weight gain, and hypotension. The balance between the wanted drug effects on psychotic symptoms and unwanted side effects are largely at play within dopaminergic brain pathways affected by haloperidol. Cortical dopamine-D2-pathways play an important role in regulating these effects and include the nigrostriatal pathway, which is responsible for causing extrapyramidal symptoms (EPS), the mesolimbic and mesocortical pathways, which are responsible for the improvement in positive schizophrenic symptoms, and the tuberoinfundibular dopamine pathway, which is responsible for hyperprolactinemia. A syndrome consisting of potentially irreversible, involuntary, dyskinetic movements may develop in patients. Although the prevalence of the syndrome appears to be highest among the elderly, especially elderly women, it is impossible to rely upon prevalence estimates to predict, at the inception of antipsychotic treatment, which patients are likely to develop the syndrome. Cases of sudden death, QT-prolongation, and Torsades de Pointes have been reported in patients receiving haloperidol. Higher than recommended doses of any formulation and intravenous administration of haloperidol appear to be associated with a higher risk of QT-prolongation and Torsades de Pointes. Although cases have been reported even in the absence of predisposing factors, particular caution is advised in treating patients with other QT-prolonging conditions (including electrolyte imbalance [particularly hypokalemia and hypomagnesemia], drugs known to prolong QT, underlying cardiac abnormalities, hypothyroidism, and familial long QT-syndrome). A potentially fatal symptom complex sometimes referred to as Neuroleptic Malignant Syndrome (NMS) has been reported in association with antipsychotic drugs. Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, altered mental status (including catatonic signs) and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac dysrhythmias). Additional signs may include elevated creatine phosphokinase, myoglobinuria (rhabdomyolysis) and acute renal failure.
Anti-Dyskinesia Agents
Drugs used in the treatment of movement disorders. Most of these act centrally on dopaminergic or cholinergic systems. Among the most important clinically are those used for the treatment of Parkinson disease (ANTIPARKINSON AGENTS) and those for the tardive dyskinesias. (See all compounds classified as Anti-Dyskinesia Agents.)
Antipsychotic Agents
Agents that control agitated psychotic behavior, alleviate acute psychotic states, reduce psychotic symptoms, and exert a quieting effect. They are used in SCHIZOPHRENIA; senile dementia; transient psychosis following surgery; or MYOCARDIAL INFARCTION; etc. These drugs are often referred to as neuroleptics alluding to the tendency to produce neurological side effects, but not all antipsychotics are likely to produce such effects. Many of these drugs may also be effective against nausea, emesis, and pruritus. (See all compounds classified as Antipsychotic Agents.)
Dopamine Antagonists
Drugs that bind to but do not activate DOPAMINE RECEPTORS, thereby blocking the actions of dopamine or exogenous agonists. Many drugs used in the treatment of psychotic disorders (ANTIPSYCHOTIC AGENTS) are dopamine antagonists, although their therapeutic effects may be due to long-term adjustments of the brain rather than to the acute effects of blocking dopamine receptors. Dopamine antagonists have been used for several other clinical purposes including as ANTIEMETICS, in the treatment of Tourette syndrome, and for hiccup. Dopamine receptor blockade is associated with NEUROLEPTIC MALIGNANT SYNDROME. (See all compounds classified as Dopamine Antagonists.)
Antiemetics
Drugs used to prevent NAUSEA or VOMITING. (See all compounds classified as Antiemetics.)
N05AD01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N05 - Psycholeptics
N05A - Antipsychotics
N05AD - Butyrophenone derivatives
N05AD01 - Haloperidol
Absorption
Haloperidol is a highly lipophilic compound and is extensively metabolized in humans, which may cause a large interindividual variability in its pharmacokinetics. Studies have found a wide variance in pharmacokinetic values for orally administered haloperidol with 1.7-6.1 hours reported for time to peak plasma concentration (tmax), 14.5-36.7 hours reported for half-life (t12), and 43.73 g/Lh [range 14.89-120.96 g/Lh] reported for AUC. Haloperidol is well-absorbed from the gastrointestinal tract when ingested orally, however, the first-pass hepatic metabolism decreases its oral bioavailability to 40 - 75%. After intramuscular administration, the time to peak plasma concentration (tmax) is 20 minutes in healthy individuals or 33.8 minutes in patients with schizophrenia, with a mean half-life of 20.7 hours. Bioavailability following intramuscular administration is higher than that for oral administration. Administration of haloperidol decanoate (the depot form of haloperidol for long-term treatment) in sesame oil results in slow release of the drug for long-term effects. The plasma concentrations of haloperidol gradually rise, reaching its peak concentration at about 6 days after the injection, with an apparent half-life of about 21 days. Steady-state plasma concentrations are achieved after the third or fourth dose.
Route of Elimination
In radiolabeling studies, approximately 30% of the radioactivity is excreted in the urine following a single oral administration of 14C-labelled haloperidol, while 18% is excreted in the urine as haloperidol glucuronide, demonstrating that haloperidol glucuronide is a major metabolite in the urine as well as in plasma in humans.
Volume of Distribution
The apparent volume of distribution was found to range from 9.5-21.7 L/kg. This high volume of distribution is in accordance with its lipophilicity, which also suggests free movement through various tissues including the blood-brain barrier.
Clearance
Following intravenous administration, the plasma or serum clearance (CL) was found to be 0.39-0.708 L/h/kg (6.5 to 11.8 ml/min/kg). Following oral administration, clearance was found to be 141.65 L/h (range 41.34 to 335.80 L/h). Haloperidol clearance after extravascular administration ranges from 0.9-1.5 l/h/kg, however this rate is reduced in poor metabolizers of C_YP2D6_ enzyme. Reduced CYP2D6 enzyme activity may result in increased concentrations of haloperidol. The inter-subject variability (coefficient of variation, %) in haloperidol clearance was estimated to be 44% in a population pharmacokinetic analysis in patients with schizophrenia. Genetic polymorphism of CYP2D6 has been demonstrated to be an important source of inter-patient variability in the pharmacokinetics of haloperidol and may affect therapeutic response and incidence of adverse effects.
Haloperidol is well absorbed from the gastrointestinal tract but first-pass hepatic metabolism decreases oral bioavailability to 40 to 75%. Serum concentration peaks 0.5 to 4 hours after an oral dose.
Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 674
The apparent volume of distribution is about 20 L/kg, consistent with the high lipophilicity of the drug. Haloperidol circulates in blood bound predominantly (90-94%) to plasma proteins.
Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 674
Following administration of haloperidol in animals, the drug is distributed mainly into the liver, with lower concentrations being distributed into the brain, lung, kidneys, spleen, and heart. ... Haloperidol is about 92% bound to plasma proteins.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 2291
Haloperidol is extensively metabolised in the liver with only about 1% of the administered dose excreted unchanged in urine. In humans, haloperidol is biotransformed to various metabolites, including p-fluorobenzoylpropionic acid, 4-(4-chlorophenyl)-4-hydroxypiperidine, reduced haloperidol, pyridinium metabolites, and haloperidol glucuronide. In psychiatric patients treated regularly with haloperidol, the concentration of haloperidol glucuronide in plasma is the highest among the metabolites, followed, in rank order, by unchanged haloperidol, reduced haloperidol and reduced haloperidol glucuronide. The drug is thought to be metabolized primarily by oxidative N-dealkylation of the piperidine nitrogen to form fluorophenylcarbonic acids and piperidine metabolites (which appear to be inactive), and by reduction of the butyrophenone carbonyl to the carbinol, forming _hydroxyhaloperidol_. The enzymes involved in the biotransformation of haloperidol include cytochrome P450 (CYP) including CYP3A4 and CYP2D6, carbonyl reductase and uridine di-phosphoglucose glucuronosyltransferase enzymes. The greatest proportion of the intrinsic hepatic clearance of haloperidol is performed by glucuronidation and followed by the reduction of haloperidol to reduced haloperidol and by CYP-mediated oxidation. In studies of cytochrome-mediated disposition in vitro, CYP3A4 appears to be the major isoform of the enzyme responsible for the metabolism of haloperidol in humans. The intrinsic clearance of the back-oxidation of reduced haloperidol to the parent compound, oxidative N-dealkylation and pyridinium formation are of the same order of magnitude. This suggests that the same enzyme system is responsible for the above three metabolic reactions. In vivo human studies on haloperidol metabolism have shown that the glucuronidation of haloperidol accounts for 50 to 60% of haloperidol biotransformation and that approximately 23% of the biotransformation was accounted for by the reduction pathway. The remaining 20 to 30% ofthe biotransformation of haloperidol would be via N-dealkylation and pyridinium formation.
Although the exact metabolic fate has not been clearly established, it appears that haloperidol is principally metabolized in the liver. The drug appears to be metabolized principally by oxidative N-dealkylation of the piperidine nitrogen to form fluorophenylcarbonic acids and piperidine metabolites (which appear to be inactive), and by reduction of the butyrophenone carbonyl to the carbinol, forming hydroxyhaloperidol. Limited data suggest that the reduced metabolite, hydroxyhaloperidol, has some pharmacologic activity, although its activity appears to be less than that of haloperidol. Urinary metabolites in rats include p-fluorophenaceturic acid, beta-p-fluorobenzoylpropionic acid, and several unidentified acids.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 2291
... it is metabolized via reduction to reduced haloperidol, which is biologically inactive. Different extents of enterohepatic recycling, and ethnic differences in metabolism, could also account for the observed variability in haloperidol disposition.
PMID:2689040 Froemming JS; Clin Pharmacokinet 17 (6): 396-423 (1989)
The enzymes involved in the biotransformation of haloperidol include cytochrome P450 (CYP), carbonyl reductase and uridine diphosphoglucose glucuronosyltransferase. The greatest proportion of the intrinsic hepatic clearance of haloperidol is by glucuronidation, followed by the reduction of haloperidol to reduced haloperidol and by CYP-mediated oxidation. In studies of CYP-mediated disposition in vitro, CYP3A4 appears to be the major isoform responsible for the metabolism of haloperidol in humans. The intrinsic clearances of the back-oxidation of reduced haloperidol to the parent compound, oxidative N-dealkylation and pyridinium formation are of the same order of magnitude, suggesting that the same enzyme system is responsible for the 3 reactions. Large variation in the catalytic activity was observed in the CYP-mediated reactions, whereas there appeared to be only small variations in the glucuronidation and carbonyl reduction pathways. Haloperidol is a substrate of CYP3A4 and an inhibitor, as well as a stimulator, of CYP2D6.
PMID:10628896 Kudo S, Ishizaki T; Clin Pharmacokinet 37 (6): 435-56 (1999)
... In vivo pharmacogenetic studies have indicated that the metabolism and disposition of haloperidol may be regulated by genetically determined polymorphic CYP2D6 activity. However, these findings appear to contradict those from studies in vitro with human liver microsomes and from studies of drug interactions in vivo. Interethnic and pharmacogenetic differences in haloperidol metabolism may explain these observations.
PMID:10628896 Kudo S, Ishizaki T; Clin Pharmacokinet 37 (6): 435-56 (1999)
Haloperidol has known human metabolites that include (2S,3S,4S,5R)-6-[4-(4-Chlorophenyl)-1-[4-(4-fluorophenyl)-4-oxobutyl]piperidin-4-yl]oxy-3,4,5-trihydroxyoxane-2-carboxylic acid, Haloperidol pyridinium, and p-Fluorobenzoylpropionic acid and 4-(4-chlorophenyl)-4-hydroxypiperidine.
Haloperidol is a known human metabolite of reduced_haloperidol.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Following oral administration, the half-life was found to be 14.5-36.7 hours. Following intramuscular injection, mean half-life was found to be 20.7 hours.
10 MG Haloperidol IV and oral administration to healthy volunteers: serum T1/2 10-19 hr after IV and 12-38.3 hr after oral administration. Bioavailability in the order of 60%; distribution volume around 1300 L.
PMID:822989 Forsman A, Ohman R; Curr Ther Res Clin Exp 20 (Sep): 319-36 (1976)
Haloperidol, Elimination: Oral: 24 hours (range 12 to 37 hours). Intramuscular: 21 hours (range, 17 to 25 hours). Intravenous: 14 hours (range, 10 to 19 hours). Haloperidol decanoate, Elimination: Approximately 3 weeks (single or multiple doses).
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1486
While haloperidol has demonstrated pharmacologic activity at a number of receptors in the brain, it exerts its antipsychotic effect through its strong antagonism of the dopamine receptor (mainly D2), particularly within the mesolimbic and mesocortical systems of the brain. Schizophrenia is theorized to be caused by a hyperdopaminergic state within the limbic system of the brain. Dopamine-antagonizing medications such as haloperidol, therefore, are thought to improve psychotic symptoms by halting this over-production of dopamine. The optimal clinical efficacy of antipsychotics is associated with the blockade of approximately 60 % - 80 % of D2 receptors in the brain. While the exact mechanism is not entirely understood, haloperidol is known to inhibit the effects of dopamine and increase its turnover. Traditional antipsychotics, such as haloperidol, bind more tightly than dopamine itself to the dopamine D2 receptor, with dissociation constants that are lower than that for dopamine. It is believed that haloperidol competitively blocks post-synaptic dopamine (D2) receptors in the brain, eliminating dopamine neurotransmission and leading to the relief of delusions and hallucinations that are commonly associated with psychosis. It acts primarily on the D2-receptors and has some effect on 5-HT2 and 1-receptors, with negligible effects on dopamine D1-receptors. The drug also exerts some blockade of -adrenergic receptors of the autonomic system. Antagonistic activity regulated through dopamine D2 receptors in the chemoreceptive trigger zone (CTZ) of the brain renders its antiemetic activity. Of the three D2-like receptors, only the D2 receptor is blocked by antipsychotic drugs in direct relation to their clinical antipsychotic abilities. Clinical brain-imaging findings show that haloperidol remains tightly bound to D2 dopamine receptors in humans undergoing 2 positron emission tomography (PET) scans with a 24h pause in between scans. A common adverse effect of this drug is the development of extrapyramidal symptoms (EPS), due to this tight binding of haloperidol to the dopamine D2 receptor. Due to the risk of unpleasant and sometimes lifelong extrapyramidal symptoms, newer antipsychotic medications than haloperidol have been discovered and formulated. Rapid dissociation of drugs from dopamine D2 receptors is a plausible explanation for the improved EPS profile of atypical antipsychotics such as [DB00734]. This is also consistent with the theory of a lower affinity for D2 receptors for these drugs. As mentioned above, haloperidol binds tightly to the dopamine receptor, potentiating the risk of extrapyramidal symptoms, and therefore should only been used when necessary.
Haloperidol has less prominent autonomic effects than do other antipsychotic drugs. It has little anticholinergic activity ... it blocks activation of alpha receptors by sympathomimetic amines but is much less potent than chlorpromazine in this action.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 166
Although the complex mechanism of the therapeutic effect is not clearly established, haloperidol is known to produce a selective effect on the central nervous system (CNS) by competitive blockade of postsynaptic dopamine (D2) receptors in the mesolimbic dopaminergic system and an increased turnover of brain dopamine to produce its tranquilizing effects. With subchronic therapy, depolarization blockade, or diminished firing rate of the dopamine neuron (decreased release) along with D2 postsynaptic blockade results in the antipsychotic action.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1456

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
93
PharmaCompass offers a list of Haloperidol API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Haloperidol manufacturer or Haloperidol supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Haloperidol manufacturer or Haloperidol supplier.
PharmaCompass also assists you with knowing the Haloperidol API Price utilized in the formulation of products. Haloperidol API Price is not always fixed or binding as the Haloperidol Price is obtained through a variety of data sources. The Haloperidol Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Haloperidol manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Haloperidol, including repackagers and relabelers. The FDA regulates Haloperidol manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Haloperidol API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Haloperidol manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Haloperidol supplier is an individual or a company that provides Haloperidol active pharmaceutical ingredient (API) or Haloperidol finished formulations upon request. The Haloperidol suppliers may include Haloperidol API manufacturers, exporters, distributors and traders.
click here to find a list of Haloperidol suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Haloperidol DMF (Drug Master File) is a document detailing the whole manufacturing process of Haloperidol active pharmaceutical ingredient (API) in detail. Different forms of Haloperidol DMFs exist exist since differing nations have different regulations, such as Haloperidol USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Haloperidol DMF submitted to regulatory agencies in the US is known as a USDMF. Haloperidol USDMF includes data on Haloperidol's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Haloperidol USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Haloperidol suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Haloperidol Drug Master File in Japan (Haloperidol JDMF) empowers Haloperidol API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Haloperidol JDMF during the approval evaluation for pharmaceutical products. At the time of Haloperidol JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Haloperidol suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Haloperidol Drug Master File in Korea (Haloperidol KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Haloperidol. The MFDS reviews the Haloperidol KDMF as part of the drug registration process and uses the information provided in the Haloperidol KDMF to evaluate the safety and efficacy of the drug.
After submitting a Haloperidol KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Haloperidol API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Haloperidol suppliers with KDMF on PharmaCompass.
A Haloperidol CEP of the European Pharmacopoeia monograph is often referred to as a Haloperidol Certificate of Suitability (COS). The purpose of a Haloperidol CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Haloperidol EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Haloperidol to their clients by showing that a Haloperidol CEP has been issued for it. The manufacturer submits a Haloperidol CEP (COS) as part of the market authorization procedure, and it takes on the role of a Haloperidol CEP holder for the record. Additionally, the data presented in the Haloperidol CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Haloperidol DMF.
A Haloperidol CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Haloperidol CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Haloperidol suppliers with CEP (COS) on PharmaCompass.
A Haloperidol written confirmation (Haloperidol WC) is an official document issued by a regulatory agency to a Haloperidol manufacturer, verifying that the manufacturing facility of a Haloperidol active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Haloperidol APIs or Haloperidol finished pharmaceutical products to another nation, regulatory agencies frequently require a Haloperidol WC (written confirmation) as part of the regulatory process.
click here to find a list of Haloperidol suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Haloperidol as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Haloperidol API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Haloperidol as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Haloperidol and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Haloperidol NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Haloperidol suppliers with NDC on PharmaCompass.
Haloperidol Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Haloperidol GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Haloperidol GMP manufacturer or Haloperidol GMP API supplier for your needs.
A Haloperidol CoA (Certificate of Analysis) is a formal document that attests to Haloperidol's compliance with Haloperidol specifications and serves as a tool for batch-level quality control.
Haloperidol CoA mostly includes findings from lab analyses of a specific batch. For each Haloperidol CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Haloperidol may be tested according to a variety of international standards, such as European Pharmacopoeia (Haloperidol EP), Haloperidol JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Haloperidol USP).
