



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
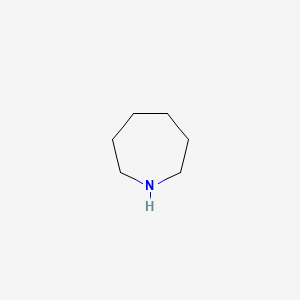

1. Hexahydroazepine
2. Hexahydroazepine Hydrochloride
3. Perhydroazepine
1. Azepane
2. 111-49-9
3. Hexahydroazepine
4. Perhydroazepine
5. Homopiperidine
6. Hexamethylenimine
7. Hexahydro-1h-azepine
8. 1h-azepine, Hexahydro-
9. Azacycloheptane
10. 1-azacycloheptane
11. Azepan
12. Cyclohexamethylenimine
13. Hexamethylene Imine
14. Azepine, Hexahydro-
15. Nsc 16236
16. Mfcd00006934
17. Czd076g73r
18. G 0
19. Chebi:32616
20. Nsc-16236
21. Dsstox_cid_6879
22. Dsstox_rid_78234
23. Dsstox_gsid_26879
24. Cycloheptane, 1-aza-
25. Cas-111-49-9
26. Hsdb 562
27. Einecs 203-875-9
28. Un2493
29. Brn 0001084
30. Unii-czd076g73r
31. Cp 18407
32. Ai3-26610
33. Ccris 8886
34. Azepane #
35. Hexamethyleneimine, 99%
36. Wln: T7mtj
37. Hexamethylene Imine (hmi)
38. Ec 203-875-9
39. 5-20-04-00003 (beilstein Handbook Reference)
40. Chembl1375444
41. Dtxsid1026879
42. Hexamethyleneimine [hsdb]
43. 2,3,4,5,6,7-hexahydroazepine
44. Act03374
45. Cs-b0958
46. Nsc16236
47. Str01425
48. Zinc3636476
49. Tox21_201482
50. Tox21_303304
51. Stl124104
52. Akos000119078
53. Un 2493
54. 2,3,4,5,6,7-hexahydro-1h-azepine
55. Ncgc00091040-01
56. Ncgc00091040-02
57. Ncgc00257117-01
58. Ncgc00259033-01
59. Bp-30036
60. H0092
61. D77224
62. Hexamethyleneimine [un2493] [flammable Liquid]
63. Q421530
64. J-002592
65. J-521455
66. Z57127566
67. F2190-0324
| Molecular Weight | 99.17 g/mol |
|---|---|
| Molecular Formula | C6H13N |
| XLogP3 | 1.2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 0 |
| Exact Mass | 99.104799419 g/mol |
| Monoisotopic Mass | 99.104799419 g/mol |
| Topological Polar Surface Area | 12 Ų |
| Heavy Atom Count | 7 |
| Formal Charge | 0 |
| Complexity | 37.4 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
HEXAMETHYLENEIMINE WAS FOUND TO BE A URINARY METABOLITE OF THE CARCINOGEN N-NITROSOHEXAMETHYLENEIMINE IN MALE RATS.
GRANDJEAN CJ; METABOLISM OF N-NITROSOHEXAMETHYLENEIMINE; J NATL CANCER INST 57(1) 181 (1976)
HEXAMETHYLENIMINE WAS FOUND TO BE A URINARY METABOLITE OF PIVMECILLINAM IN HUMANS.
HATTORI M ET AL; METABOLITES OF PIVMECILLINAM IN HUMAN URINE; CHEMOTHERAPY (TOKYO) 25(1) 123 (1977)


