
Synopsis
Synopsis
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDA Orange Book

0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz

1. Bactidol
2. Doreperol
3. Duranil
4. Elsix
5. Hexigel
6. Hexoral
7. Hextril
8. Oraldene
9. Oraldine
10. Steri-sol
1. 141-94-6
2. Oraldene
3. Sterisil
4. Hexoral
5. Glypesin
6. Hextril
7. Elsix
8. Sterilate
9. Triocil
10. Triscol
11. Collu Hextril
12. Duranil Aerosol
13. Hexetidinum
14. Steri/sol
15. Nsc-17764
16. 1,3-bis(2-ethylhexyl)-5-methyl-1,3-diazinan-5-amine
17. 5-amino-1,3-bis(2-ethylhexyl)hexahydro-5-methylpyrimidine
18. Nsc 17764
19. 5-pyrimidinamine, 1,3-bis(2-ethylhexyl)hexahydro-5-methyl-
20. Hexetidine, Mixture Of Stereoisomers
21. Mls002207232
22. 1,3-bis(2-ethylhexyl)-5-methylhexahydropyrimidin-5-amine
23. 5-amino-1,3-bis(2-ethylhexyl)-5-methylhexahydropyrimidine
24. 852a84y8ls
25. Hexetidine (inn)
26. Drossadin
27. Hexetidina
28. Oraseptic
29. Paradenyl
30. Hexetidine 100 Microg/ml In Acetonitrile
31. Pyrimidine, 5-amino-1,3-bis(2-ethylhexyl)hexahydro-5-methyl-
32. Hexetidine [inn]
33. Collu-hextril
34. Dsstox_cid_25297
35. Dsstox_rid_80788
36. Dsstox_gsid_45297
37. Steri/sol (van)
38. Hexigel
39. Caswell No. 033bb
40. Hexetidinum [inn-latin]
41. Hexetidina [inn-spanish]
42. 5-amino-1,3-bis(2-ethylhexyl)hexahydro-5-methylpyrimidine, Mix Of Diastereomers
43. Hexetidine [inn:ban]
44. Sr-01000872662
45. Einecs 205-513-5
46. Hexopyrimidine
47. Esetidina
48. Hexetidin
49. Hexocil
50. Unii-852a84y8ls
51. Ai3-15546
52. P 252
53. Hsdb 7828
54. Ncgc00016404-01
55. Cas-141-94-6
56. Prestwick_800
57. Spectrum_000236
58. 1,3-bis(2-ethylhexyl)-5-amino-hexahydro-5-methylpyrimidin
59. Hexetidine [mi]
60. Prestwick0_000551
61. Prestwick1_000551
62. Prestwick2_000551
63. Prestwick3_000551
64. Spectrum2_000953
65. Spectrum3_000271
66. Spectrum4_000395
67. Spectrum5_001435
68. Hexetidine [hsdb]
69. Hexetidine [inci]
70. Hexetidine [mart.]
71. Hexetidine [who-dd]
72. Schembl56672
73. Bspbio_000621
74. Bspbio_001742
75. Kbiogr_000949
76. Kbioss_000716
77. Divk1c_000280
78. Spectrum1500633
79. Spbio_000946
80. Spbio_002542
81. Bpbio1_000685
82. Chembl144673
83. Dtxsid1045297
84. Chebi:94339
85. Hms500n22
86. Kbio1_000280
87. Kbio2_000716
88. Kbio2_003284
89. Kbio2_005852
90. Kbio3_001242
91. 1,3-bis(2-ethylhexyl)hexahydro-5-methyl-5-pyrimidiamine
92. 5-amino-1,3-bis(2-ethylhexyl)-5-methyl-hexhydropyrimidine
93. Hexetidine [ep Monograph]
94. Ninds_000280
95. Hms1569p03
96. Hms1921m09
97. Hms2096p03
98. Hms3713p03
99. Pharmakon1600-01500633
100. Hy-b0996
101. Nsc17764
102. Tox21_110424
103. Ccg-39752
104. Mfcd00010428
105. Nsc757394
106. S6408
107. Akos000277944
108. Akos024348907
109. Tox21_110424_1
110. Cs-4490
111. Db08958
112. Nsc-757394
113. Idi1_000280
114. Ncgc00094819-01
115. Ncgc00094819-02
116. Ncgc00094819-03
117. Ncgc00094819-04
118. Ncgc00094819-07
119. As-56257
120. Smr000059080
121. Hexetidine, Mixture Of Stereoisomers, 97%
122. Sbi-0051566.p002
123. Db-042594
124. Ab00052133
125. Ft-0627047
126. D07068
127. D94751
128. Ab00052133_06
129. 141h946
130. Pyrimidine,3-bis(2-ethylhexyl)hexahydro-5-methyl-
131. Q419749
132. Sr-01000872662-1
133. Sr-01000872662-2
134. 5-pyrimidinamine,3-bis(2-ethylhexyl)hexahydro-5-methyl-
135. 5-pyrimidinamine,1,3-bis(2-ethylhexyl)hexahydro-5-methyl-
136. Hexetidine, European Pharmacopoeia (ep) Reference Standard
137. 1,3-bis(2-ethylhexyl)-5-methylhexahydro-5-pyrimidinamine #
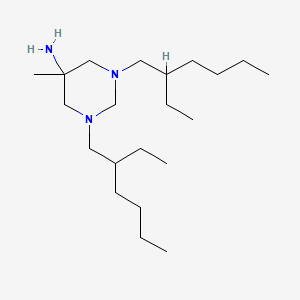
| Molecular Weight | 339.6 g/mol |
|---|---|
| Molecular Formula | C21H45N3 |
| XLogP3 | 5.7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 12 |
| Exact Mass | 339.361348448 g/mol |
| Monoisotopic Mass | 339.361348448 g/mol |
| Topological Polar Surface Area | 32.5 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 292 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 2 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
A bactericidal and fungicidal antiseptic. It is used as a 0.1% mouthwash for local infections and oral hygiene.
Natiional Library of Medicine; MeSH Heading for hexetidine. Available from, as of August 6, 2010: https://www.nlm.nih.gov/cgi/mesh/2010/MB_cgi?term=Hexetidine
MEDICATION (VET): Hexetidine is a cationic antiseptic with a wide spectrum of actions against Gram-positive and Gram-negative bacteria, as well as some fungi and parasites. In veterinary medicine, it is indicated for use in horses as a shampoo, at low concentrations, for topical application to the skin. The use of hexetidine as teat disinfectant, both as teat dip and teat spray, is also known.
European Medicines Agency (EMA), The European Agency for the Evaluation of Medicinal Products, Veterinary Medicines Evaluation Unit, Committee for Veterinary Medicinal Products; Hexetidine, Summary Report (April 1998). Available from, as of July 14, 2010: https://www.ema.europa.eu/pdfs/vet/mrls/038298en.pdf
Hexetidine is a nonantibiotic antimicrobial agent that possesses broad-spectrum antimicrobial activity against Gram-positive and Gram-negative bacteria and fungi such as Candida albicans. Several studies have identified the antiplaque activity of hexetidine. Hexetidine has been shown to be effective against isolates of Staphylococcus aureus and Pseudomonas aeruginosa in planktonic form and against biofilms of the same microorganisms on PVC. Hexetidine has also been reported to reduce the adherence of Candida albicans to human buccal epithelial cells in vitro. Hexetidine has been shown to be a promising candidate antimalarial agent with IC50 values being comparable with those of quinine chlorohydrate and chloroquine sulfate.
Table: Minimum Inhibitory Concentrations (MICs) for Hexetidine [Table#7543]
Rowe, R.C., Sheskey, P.J., Quinn, M.E.; (Eds.), Handbook of Pharmaceutical Excipients 6th edition Pharmaceutical Press, London, England 2009, p. 304-5
This examiner-blind, parallel group, controlled clinical study examined the effectiveness of a hexetidine (0.1%) mouthwash both in inhibiting the development of supragingival plaque and in reducing gingivitis. One hundred and thirty-four adult subjects completed the 2-week experimental gingivitis model study. Following baseline examinations, which included plaque index, modified gingival index and gingival bleeding index, subjects received a full dental prophylaxis. Subjects were randomly assigned to one of three mouthwashes (hexetidine 0.1%, chlorhexidine 0.12% (positive control) or a 5% hydroalcohol negative control) and commenced three times daily supervised rinsing as their sole method of oral hygiene. All indices were rescored after 2 weeks. Compared to the negative control group, the hexetidine group demonstrated a statistically significant inhibition and reduction of supragingival plaque and gingival inflammation with reductions of 6.3%, 33.5% and 56% for gingivitis, plaque and gingival bleeding, respectively. The results of the chlorhexidine group were used to validate the study. The study confirms the efficacy of a hexetidine rinse in reducing supragingival plaque and gingival inflammation.
PMID:12834495 Sharma NC et al; J Clin Periodontol 30 (7): 590-4 (2003)
For more Therapeutic Uses (Complete) data for Hexetidine (8 total), please visit the HSDB record page.
Oral disinfectants containing chlorhexidine or hexetidine are able to produce disturbances of taste, as demonstrated by Krarup's electrogustometric method and the gustometric method of Harris and Kalmus. Hypo- and dysgeusia are characterized by dissociated disturbances, the most prominent of which concerns the sweet perception. The bitter taste is least affected, whereas the effects on salty and acidic tastes range between that for sweet and bitter. Taste disturbances which include ageusia for 48 hr were observed when the tongue was touched with a 20% solution of chlorhexidine. Not only the disinfectants themselves provoked dysgeusia but also other "taste improving" agents (particularly, the volatile /oils/). In addition to dysgeusia, the authors found disturbances of the mucous membrane sensitivity caused by the test substances.
PMID:711516 Schaupp H, Wohnaut H; HNO 26 (10): 335-41 (1978)
The study in situ measured enamel erosion by acidified sodium chlorite, essential oil and hexetidine mouthrinses over 15-day study periods. The study was a 5 treatment, single blind cross over design involving 15 healthy subjects using orange juice, as a drink, and water, as a rinse, as positive and negative controls respectively. 2 enamel specimens from unerupted human third molar teeth were placed in the palatal area of upper removable acrylic appliances which were worn from 9 a.m. to 5 p.m., Monday to Friday for 3 weeks. Rinses were used 2x daily and 250 ml volumes of orange juice were imbibed 4x daily. Enamel loss was determined by profilometry on days 5, 10 and 15. The study in vitro involved immersing specimens in the 4 test solutions together with a reduced acid acidified sodium chlorite formulation for a period of 4 hr under constant stirring; Enamel loss was measured by profilometry every hour. Enamel loss was in situ progressive over time with the 3 rinses and orange juice but negligible with water. Acidified sodium chlorite produced similar erosion to orange juice and significantly more than the two proprietary rinses and water. The essential oil and hexetidine rinses produced similar erosion and significantly more than water. Enamel loss in vitro was progressive over time, and the order from low to high erosion was reduced acid acidified sodium chlorite, acidified sodium chlorite, Essential oil, and hexetidine mouthrinses and orange juice. Based on the study in situ, it is recommended that low pH mouthrinses should not be considered for long term or continuous use and never as pre-brushing rinses. In view of the plaque inhibitory efficacy of acidified sodium chlorite, short- to medium-term applications similar to those of chlorhexidine would be envisaged.
PMID:11314887 Pontefract H et al; J Clin Periodontol 28 (4): 319-24 (2001)
Hexetidine has been used in human medicine as an antiseptic agent for over 40 years. Although, mild buccal irritation has been reported on rare occasions, there is no evidence of any significant toxic effect from hexetidine in man (eg, if swallowed when being used as a mouthwash).
European Medicines Agency (EMA), The European Agency for the Evaluation of Medicinal Products, Veterinary Medicines Evaluation Unit, Committee for Veterinary Medicinal Products; Hexetidine, Summary Report (April 1998). Available from, as of July 14, 2010: https://www.ema.europa.eu/pdfs/vet/mrls/038298en.pdf
In a few individuals mild irritation (described as sore mouth, burning or itching) of the tongue and/or buccal tissues has been reported. Other side effects which are reported very rarely include transient anesthesia and taste impairment.
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Oraldene (contains 0.1% w/v hexetidine), (Last updated December 2009). Available from, as of July 14, 2010: https://www.medicines.org.uk/EMC/medicine/7181/SPC/Oraldene/
It is not known whether hexetidine is excreted in human breast milk, however, in view of the negligible amount of hexetidine which could be predicted to be systemically absorbed, it is unlikely that concentrations of hexetidine in the milk will present any risk to the neonate/infant.
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Oraldene (contains 0.1% w/v hexetidine), (Last updated December 2009). Available from, as of July 14, 2010: https://www.medicines.org.uk/EMC/medicine/7181/SPC/Oraldene/
Anti-Infective Agents, Local
Substances used on humans and other animals that destroy harmful microorganisms or inhibit their activity. They are distinguished from DISINFECTANTS, which are used on inanimate objects. (See all compounds classified as Anti-Infective Agents, Local.)
Antifungal Agents
Substances that destroy fungi by suppressing their ability to grow or reproduce. They differ from FUNGICIDES, INDUSTRIAL because they defend against fungi present in human or animal tissues. (See all compounds classified as Antifungal Agents.)
A01AB12
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
A - Alimentary tract and metabolism
A01 - Stomatological preparations
A01A - Stomatological preparations
A01AB - Antiinfectives and antiseptics for local oral treatment
A01AB12 - Hexetidine
G - Genito urinary system and sex hormones
G01 - Gynecological antiinfectives and antiseptics
G01A - Antiinfectives and antiseptics, excl. combinations with corticosteroids
G01AX - Other antiinfectives and antiseptics
G01AX16 - Hexetidine
Because of its cationic nature, hexetidine is absorbed to the mucous membranes and dental plaque after oral administration and is not easily removed. Studies in human beings with radiolabeled hexetidine have shown that it is retained on buccal tissues for 8 to 10 hours after a single oral rinse and it has been possible to detect the continued presence of it on the oral tissues for as long as 65 hours after application.
European Medicines Agency (EMA), The European Agency for the Evaluation of Medicinal Products, Veterinary Medicines Evaluation Unit, Committee for Veterinary Medicinal Products; Hexetidine, Summary Report (April 1998). Available from, as of July 14, 2010: https://www.ema.europa.eu/pdfs/vet/mrls/038298en.pdf

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
36
PharmaCompass offers a list of Hexetidine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Hexetidine manufacturer or Hexetidine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Hexetidine manufacturer or Hexetidine supplier.
PharmaCompass also assists you with knowing the Hexetidine API Price utilized in the formulation of products. Hexetidine API Price is not always fixed or binding as the Hexetidine Price is obtained through a variety of data sources. The Hexetidine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Hexetidine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Hexetidine, including repackagers and relabelers. The FDA regulates Hexetidine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Hexetidine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Hexetidine manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Hexetidine supplier is an individual or a company that provides Hexetidine active pharmaceutical ingredient (API) or Hexetidine finished formulations upon request. The Hexetidine suppliers may include Hexetidine API manufacturers, exporters, distributors and traders.
click here to find a list of Hexetidine suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Hexetidine DMF (Drug Master File) is a document detailing the whole manufacturing process of Hexetidine active pharmaceutical ingredient (API) in detail. Different forms of Hexetidine DMFs exist exist since differing nations have different regulations, such as Hexetidine USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Hexetidine DMF submitted to regulatory agencies in the US is known as a USDMF. Hexetidine USDMF includes data on Hexetidine's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Hexetidine USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Hexetidine suppliers with USDMF on PharmaCompass.
A Hexetidine CEP of the European Pharmacopoeia monograph is often referred to as a Hexetidine Certificate of Suitability (COS). The purpose of a Hexetidine CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Hexetidine EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Hexetidine to their clients by showing that a Hexetidine CEP has been issued for it. The manufacturer submits a Hexetidine CEP (COS) as part of the market authorization procedure, and it takes on the role of a Hexetidine CEP holder for the record. Additionally, the data presented in the Hexetidine CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Hexetidine DMF.
A Hexetidine CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Hexetidine CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Hexetidine suppliers with CEP (COS) on PharmaCompass.
Hexetidine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Hexetidine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Hexetidine GMP manufacturer or Hexetidine GMP API supplier for your needs.
A Hexetidine CoA (Certificate of Analysis) is a formal document that attests to Hexetidine's compliance with Hexetidine specifications and serves as a tool for batch-level quality control.
Hexetidine CoA mostly includes findings from lab analyses of a specific batch. For each Hexetidine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Hexetidine may be tested according to a variety of international standards, such as European Pharmacopoeia (Hexetidine EP), Hexetidine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Hexetidine USP).
