
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
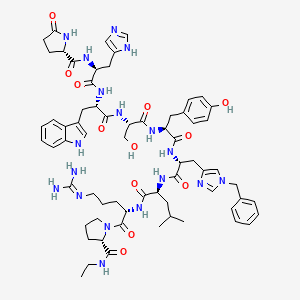

1. ((im Bzl)-d-his(6), Pro(9)-net)lhrh
2. 6-his(imbzl)-9-n-et-pronh2-10-des-glynh2-lhrh
3. Gnrh, His(imbzl)(6)-n-et-pronh2(9)-
4. Ibhpe-lhrh
5. Imbzl-his(6), Pro(9)-net-gnrh
6. Lhrh, His(imbzl)(6)-n-et-pronh2(9)-
7. Lhrh, Histidyl(imbzl)(6)-n-ethylprolinamide(9)-des-glycinamide(10)-
8. Supprelin
1. 76712-82-8
2. Histrelin Acetate
3. Histrelinum
4. Orf 17070
5. Rwj 17070
6. Histrelina
7. Histreline
8. Vantas
9. Orf-17070
10. Chebi:5739
11. H50h3s3w74
12. Rwj-17070
13. Orf 17070rwj 17070
14. Vantaas
15. Vantas (tn)
16. Luteinizing Hormone-releasing Factor (pig), 6-(1-(phenylmethyl)-d-histidine)-9-(n-ethyl-l-prolinamide)-10-deglycinamide-
17. Histreline [french]
18. Histrelinum [latin]
19. Histrelina [spanish]
20. [(im-bzl)-d-his(6),pro(9)-net]-gonadotropin Releasing Hormone
21. Histrelin [usan:inn]
22. 1-9-luteinizing Hormone-releasing Factor (swine),6-[1-(phenylmethyl)-d-histidine]-9-(n-ethyl-l-prolinamide)-
23. 5-oxo-l-prolyl-l-histidyl-l-tryptophyl-l-seryl-l-tyrosyl-1-benzyl-d-histidyl-l-leucyl-l-arginyl-n-ethyl-l-prolinamide
24. 5-oxo-l-prolyl-l-histidyl-l-tryptophyl-l-seryl-l-tyrosyl-n(tau)-benzyl-d-histidyl-l-leucyl-l-arginyl-n-ethyl-l-prolinamide
25. L-pyroglutamyl-l-histidyl-l-tryptophyl-l-seryl-l-tyrosyl-d-n(im)-benzyl-histidyl-l-leucyl-l-arginyl-l-proline Ethylamide
26. 220810-26-4
27. Brn 4290557
28. Unii-h50h3s3w74
29. Hsdb 7657
30. ((imbl)-d-his(sub 6),pro(sub 9)-net)lhrh
31. Histrelin [inn]
32. Histrelin [mi]
33. Histrelin (usan/inn)
34. Histrelin [hsdb]
35. Histrelin [usan]
36. Supprelin Lasupprelin La
37. Histrelin [vandf]
38. Histrelin [mart.]
39. Histrelin [who-dd]
40. Schembl17881
41. 5-oxo-l-prolyl-l-histidyl-l-tryptophyl-l-seryl-l-tyrosyl-n(sup Tau)-benzyl-d-histidyl-l-leucyl-l-arginyl-n-ethyl-l-prolinamide
42. Gtpl3884
43. Chembl1201255
44. Schembl19409305
45. Schembl19712198
46. Schembl22288995
47. Dtxsid50227543
48. Hy-p0056
49. Ncgc00181758-01
50. Luteinizing Hormone-releasing Factor, 6-(1-(phenylmethyl)-d-histidine)-9-(n-ethyl-l-prolinamide)-10-deglycinamide-
51. Des-gly10,(d-his(bzl)6)-lh-rh*ethylamide
52. Cs-0015085
53. (des-gly10,d-his(bzl)6,pro-nhet9)-lhrh
54. D02369
55. 712h828
56. Q5871149
57. 5-oxo-l-prolyl-l-histidyl-l-tryptophyl-l-seryl-l-tyrosyl-nt-benzyl-d-histidyl-l-leucyl-l-arginyl-n-ethyl-l-prolinamide
58. L-prolinamide, 5-oxo-l-prolyl-l-histidyl-l-tryptophyl-l-seryl-l-tyrosyl-1-(phenylmethyl)-d-histidyl-l-leucyl-l-arginyl-n-ethyl-
| Molecular Weight | 1323.5 g/mol |
|---|---|
| Molecular Formula | C66H86N18O12 |
| XLogP3 | 0.7 |
| Hydrogen Bond Donor Count | 15 |
| Hydrogen Bond Acceptor Count | 15 |
| Rotatable Bond Count | 34 |
| Exact Mass | 1322.66726025 g/mol |
| Monoisotopic Mass | 1322.66726025 g/mol |
| Topological Polar Surface Area | 449 Ų |
| Heavy Atom Count | 96 |
| Formal Charge | 0 |
| Complexity | 2660 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 9 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Supprelin la |
| PubMed Health | Histrelin (Implantation) |
| Drug Classes | Endocrine-Metabolic Agent |
| Active Ingredient | Histrelin acetate |
| Dosage Form | Implant |
| Route | Subcutaneous |
| Strength | 50mg |
| Market Status | Prescription |
| Company | Endo Pharm |
| 2 of 4 | |
|---|---|
| Drug Name | Vantas |
| PubMed Health | Histrelin (Implantation) |
| Drug Classes | Endocrine-Metabolic Agent |
| Active Ingredient | Histrelin acetate |
| Dosage Form | Implant |
| Route | Subcutaneous |
| Strength | 50mg |
| Market Status | Prescription |
| Company | Endo Pharm |
| 3 of 4 | |
|---|---|
| Drug Name | Supprelin la |
| PubMed Health | Histrelin (Implantation) |
| Drug Classes | Endocrine-Metabolic Agent |
| Active Ingredient | Histrelin acetate |
| Dosage Form | Implant |
| Route | Subcutaneous |
| Strength | 50mg |
| Market Status | Prescription |
| Company | Endo Pharm |
| 4 of 4 | |
|---|---|
| Drug Name | Vantas |
| PubMed Health | Histrelin (Implantation) |
| Drug Classes | Endocrine-Metabolic Agent |
| Active Ingredient | Histrelin acetate |
| Dosage Form | Implant |
| Route | Subcutaneous |
| Strength | 50mg |
| Market Status | Prescription |
| Company | Endo Pharm |
Gonadotropin-Releasing Hormone agonist
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
/Histrelin/ is indicated in the palliative treatment of advanced prostate cancer. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for Vantas (Histrelin acetate) (Implant) (May 2008). Available from, as of November 9, 2008: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=7362
/Histrelin/ is a gonadotropin releasing hormone (GnRH) agonist indicated for the treatment of children with central precocious puberty (CPP). /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for Supprelin LA (Histrelin acetate) (Implant) (August 2008). Available from, as of November 9, 2008: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=8125
/Histrelin is contraindicated in patients with/ known hypersensitivity to histrelin or any ingredient in the formulation, other gonadotropin-releasing hormone (GnRH) agonists, or GnRH.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 1082
Like other GnRH agonists, histrelin causes a transient increase in serum testosterone concentrations during the first week of treatment. Worsening of signs and/or symptoms of prostate cancer and/or development of new manifestations (e.g., bone pain, neuropathy, hematuria, ureteral or bladder outlet obstruction) may occur during the first few weeks of therapy.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 1082
Cases of ureteral obstruction and spinal cord compression, which may contribute to paralysis with or without fatal complications, have been reported with GnRH agonists. Patients with metastatic vertebral lesions and/or urinary tract obstruction should be closely observed during the first few weeks of therapy. If spinal cord compression or renal impairment develops, standard treatment of these complications should be instituted.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 1082
Anaphylactic reactions have been reported with synthetic gonadotropin-releasing hormone (GnRH) or GnRH agonists.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 1082
For more Drug Warnings (Complete) data for HISTRELIN (16 total), please visit the HSDB record page.
L - Antineoplastic and immunomodulating agents
L02 - Endocrine therapy
L02A - Hormones and related agents
L02AE - Gonadotropin releasing hormone analogues
L02AE05 - Histrelin
Histrelin acetate is not active when given orally.
US Natl Inst Health; DailyMed. Current Medication Information for Vantas (Histrelin acetate) (Implant) (May 2008). Available from, as of November 9, 2008: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=7362
Following subcutaneous insertion of a histrelin acetate implant in patients with advanced prostate cancer, peak serum concentrations of histrelin occurred at a median of 12 hours; the drug is delivered continuously at a rate of 50-60 ug daily over 12 months.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 1083
Following subcutaneous insertion of one Vantas (histrelin implant) 50 mg implant in advanced prostate cancer patients (n = 17), peak serum concentrations of 1.10 +/- 0.375 ng/mL (mean +/- SD) occurred at a median of 12 hours.
US Natl Inst Health; DailyMed. Current Medication Information for Vantas (Histrelin acetate) (Implant) (May 2008). Available from, as of November 9, 2008: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=7362
The average rate of subcutaneous drug release from 41 implants assayed for residual drug content was 56.7 +/- 7.71 ug/day over the 52 week dosing period.
US Natl Inst Health; DailyMed. Current Medication Information for Vantas (Histrelin acetate) (Implant) (May 2008). Available from, as of November 9, 2008: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=7362
For more Absorption, Distribution and Excretion (Complete) data for HISTRELIN (8 total), please visit the HSDB record page.
An in vitro drug metabolism study using human hepatocytes identified a single histrelin metabolite resulting from C-terminal dealkylation. Peptide fragments resulting from hydrolysis are also likely metabolites.
US Natl Inst Health; DailyMed. Current Medication Information for Vantas (Histrelin acetate) (Implant) (May 2008). Available from, as of November 9, 2008: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=7362
The mean terminal half-life of the drug following subcutaneous injection of a single 500-ug dose was approximately 3.92 hours in healthy men.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 1083
Histrelin acetate is a synthetic nonapeptide analog of gonadotropin-releasing hormone (GnRH, luteinizing hormone-releasing hormone [LHRH], gonadorelin).
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 1083
Histrelin is a potent inhibitor of gonadotropin secretion when given continuously in therapeutic doses. After initial administration of histrelin, there is a transient surge in circulating concentrations of luteinizing hormone (LH), follicle-stimulating hormone (FSH), and gonadal steroids (testosterone and dihydrotestosterone in males). Following chronic and continuous administration of histrelin (generally, 2-4 weeks after initiation of therapy), a sustained decrease in LH and FSH secretion and a marked reduction in serum testosterone concentrations are observed. Reductions in serum testosterone concentrations in males receiving histrelin are comparable to those achieved after surgical castration (i.e., less than 50 ng/dL).
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 1083
Histrelin acetate, an LH-RH agonist, acts as a potent inhibitor of gonadotropin secretion when given continuously in therapeutic doses. Both animal and human studies indicate that following an initial stimulatory phase, chronic, subcutaneous administration of histrelin acetate desensitizes responsiveness of the pituitary gonadotropin which, in turn, causes a reduction in testicular steroidogenesis. In humans, administration of histrelin acetate results in an initial increase in circulating levels of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), leading to a transient increase in concentration of gonadal steroids (testosterone and dihydrotestosterone in males). However, continuous administration of histrelin acetate results in decreased levels of LH and FSH. In males, testosterone is reduced to castrate levels. These decreases occur within 2 to 4 weeks after initiation of treatment.
US Natl Inst Health; DailyMed. Current Medication Information for Vantas (Histrelin acetate) (Implant) (May 2008). Available from, as of November 9, 2008: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=7362
The possible direct effect of gonadotropin-releasing hormone (GnRH) and a potent GnRH agonist [( imBzl )-D- His6 -Pro9-NEt]-GnRH on basal and human chorionic gonadotropin (hCG)-stimulated progesterone, androstenedione, and estradiol production by cultured human luteal cells was examined. Luteal cells from the early or midluteal phase of the menstrual cycle responded to hCG stimulation with two to fivefold increases in steroid production in both short-term (4 hours) and long-term (up to 144 hours) culture in chemically defined medium without serum. After 48 hours in this system, levels of androstenedione and estradiol were very low, and progesterone was the predominant steroid produced. The addition of GnRH or a potent GnRH agonist to the medium had no effect on either basal or hCG-stimulated steroid secretion. When luteal cells were cultured longer (for up to 10 days) in the presence of serum, GnRH agonist caused no significant alteration of either basal or hCG-stimulated progesterone production. Collectively, these results support the conclusion that GnRH and its potent agonist do not act directly on human corpora luteal cells to modulate steroidogenesis.
PMID:6373386 Casper RF et al; Fertil Steril 42 (1): 39-43 (1984)
ABOUT THIS PAGE
97
PharmaCompass offers a list of Histrelin API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Histrelin manufacturer or Histrelin supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Histrelin manufacturer or Histrelin supplier.
PharmaCompass also assists you with knowing the Histrelin API Price utilized in the formulation of products. Histrelin API Price is not always fixed or binding as the Histrelin Price is obtained through a variety of data sources. The Histrelin Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Histrelin manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Histrelin, including repackagers and relabelers. The FDA regulates Histrelin manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Histrelin API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Histrelin manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Histrelin supplier is an individual or a company that provides Histrelin active pharmaceutical ingredient (API) or Histrelin finished formulations upon request. The Histrelin suppliers may include Histrelin API manufacturers, exporters, distributors and traders.
click here to find a list of Histrelin suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Histrelin DMF (Drug Master File) is a document detailing the whole manufacturing process of Histrelin active pharmaceutical ingredient (API) in detail. Different forms of Histrelin DMFs exist exist since differing nations have different regulations, such as Histrelin USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Histrelin DMF submitted to regulatory agencies in the US is known as a USDMF. Histrelin USDMF includes data on Histrelin's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Histrelin USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Histrelin suppliers with USDMF on PharmaCompass.
Histrelin Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Histrelin GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Histrelin GMP manufacturer or Histrelin GMP API supplier for your needs.
A Histrelin CoA (Certificate of Analysis) is a formal document that attests to Histrelin's compliance with Histrelin specifications and serves as a tool for batch-level quality control.
Histrelin CoA mostly includes findings from lab analyses of a specific batch. For each Histrelin CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Histrelin may be tested according to a variety of international standards, such as European Pharmacopoeia (Histrelin EP), Histrelin JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Histrelin USP).
