

Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
API
0
FDF
0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
US Medicaid
NA
Finished Drug Prices
NA
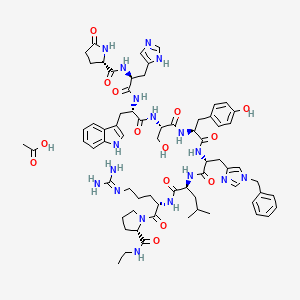

1. 220810-26-4
2. Vantas
3. Histrelin (acetate)
4. Schembl18715
5. Chembl1200509
6. Dtxsid30176580
7. Mfcd00918679
8. 767h828
9. (des-gly10,d-his(bzl)6,pro-nhet9)-lhrh Acetate
| Molecular Weight | 1383.6 g/mol |
|---|---|
| Molecular Formula | C68H90N18O14 |
| Hydrogen Bond Donor Count | 16 |
| Hydrogen Bond Acceptor Count | 17 |
| Rotatable Bond Count | 34 |
| Exact Mass | 1382.68838962 g/mol |
| Monoisotopic Mass | 1382.68838962 g/mol |
| Topological Polar Surface Area | 487 Ų |
| Heavy Atom Count | 100 |
| Formal Charge | 0 |
| Complexity | 2690 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 9 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Vantas |
| PubMed Health | histrelin |
| Drug Classes | Endocrine-Metabolic Agent |
| Active Ingredient | Histrelin acetate |
| Dosage Form | Implant |
| Route | Subcutaneous |
| Strength | 50mg |
| Market Status | Prescription |
| Company | Endo Pharm |
| 2 of 2 | |
|---|---|
| Drug Name | Vantas |
| PubMed Health | histrelin |
| Drug Classes | Endocrine-Metabolic Agent |
| Active Ingredient | Histrelin acetate |
| Dosage Form | Implant |
| Route | Subcutaneous |
| Strength | 50mg |
| Market Status | Prescription |
| Company | Endo Pharm |
 Bachem Group is a public, innovation-driven company specializing in the development and manufacturing of peptides and oligonucleotides.
Bachem Group is a public, innovation-driven company specializing in the development and manufacturing of peptides and oligonucleotides.
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 16997
Submission : 2003-10-30
Status : Active
Type : II
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : SUPPRELIN
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : EQ 0.2MG BASE/ML
Approval Date : 1991-12-24
Application Number : 19836
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : SUPPRELIN
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : EQ 0.5MG BASE/ML
Approval Date : 1991-12-24
Application Number : 19836
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : SUPPRELIN
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : EQ 1MG BASE/ML
Approval Date : 1991-12-24
Application Number : 19836
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

Brand Name : VANTAS
Dosage Form : IMPLANT;SUBCUTANEOUS
Dosage Strength : 50MG
Approval Date : 2004-10-12
Application Number : 21732
RX/OTC/DISCN : DISCN
RLD : Yes
TE Code :

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Brand Name : SUPPRELIN LA
Dosage Form : IMPLANT;SUBCUTANEOUS
Dosage Strength : 50MG
Approval Date : 2007-05-03
Application Number : 22058
RX/OTC/DISCN : RX
RLD : Yes
TE Code :

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Related Excipient Companies

Excipients by Applications
Global Sales Information

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
