
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
KDMF
0
VMF
0
FDF
0
FDA Orange Book

0
Europe

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
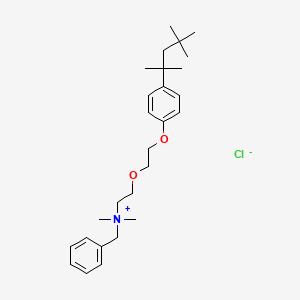

1. Bencetonium Chloride
2. Benzethonium
3. Chloride, Bencetonium
4. Chloride, Benzethonium
5. Formula Magic
6. Hyamine 1622
7. Magic, Formula
8. Orchid Fresh Ii
9. Phemeride
10. Phemerol
11. Phemethryn
12. Puri Clens
13. Puri-clens
14. Puriclens
15. Quatrachlor
16. Solamin
1. 121-54-0
2. Hyamine
3. Phemeride
4. Phemerol Chloride
5. Quatrachlor
6. Benzethoniumchloride
7. Hyamine 1622
8. Banagerm
9. Phemerol
10. Phemithyn
11. Disilyn
12. Kylacol
13. Diapp
14. Polymine D
15. Benzetonium Chloride
16. Anti-germ 77
17. Benzethonii Chloridum
18. Antiseptol
19. Cloruro De Benzetonio
20. Inactisol
21. Neostelin Green
22. Chlorure De Benzethonium
23. Sanizol
24. Benzethonium Chloride 1622
25. Microklenz
26. P-tert-octylphenoxyethoxyethyldimethylbenzylammonium Chloride
27. Nsc-20200
28. 5929-09-9
29. Benzyl-dimethyl-[2-[2-[4-(2,4,4-trimethylpentan-2-yl)phenoxy]ethoxy]ethyl]azanium;chloride
30. Diisobutylphenoxyethoxyethyl Dimethyl Benzyl Ammonium Chloride
31. Diisobutylphenoxyethoxyethyldimethyl Benzyl Ammonium Chloride
32. Chebi:31264
33. Phemerol Chloride Monohydrate
34. N-benzyl-n,n-dimethyl-2-(2-(4-(2,4,4-trimethylpentan-2-yl)phenoxy)ethoxy)ethanaminium Chloride
35. Ph41d05744
36. 1313-08-2
37. Benzenemethanaminium, N,n-dimethyl-n-(2-(2-(4-(1,1,3,3-tetramethylbutyl)phenoxy)ethoxy)ethyl)-, Chloride
38. P-diisobutyl Phenoxyethoxyethyl Dimethyl Benzylammonium Chloride
39. (2-(2-(4-diisobutylphenoxy)ethoxy)ethyl)dimethylbenzylammonium Chloride
40. Dsstox_cid_3810
41. 121-54-0 (cl-)
42. Benzyldimethyl-p-(1,1,3,3-tetramethylbutyl)phenoxyethoxy-ethylammonium Chloride
43. Benzetonio Cloruro
44. Benzyldimethyl(2-(2-(p-(1,1,3,3-tetramethylbutyl)phenoxy)ethoxy)ethyl)ammonium Chloride
45. Dsstox_rid_77195
46. Dsstox_gsid_23810
47. Benzethoni Chloridum
48. N,n-dimethyl-n-(2-(2-(4-(1,1,3,3-tetramethylbutyl)phenoxy)ethoxy)ethyl)-benzenemethanaminium Chloride
49. N-benzyl-n,n-dimethyl-2-(2-(4-(2,4,4-trimethylpentan-2-yl)phenoxy)ethoxy)ethan-1-aminium Chloride
50. Qac
51. P-diisobutyl(phenoxyethoxy)ethyl]dimethylbenzylammonium Chloride
52. Chembl221753
53. Rs89wu8v92
54. Benzyldimethyl(2-(2-(4-(1,1,3,3-tetramethylbutyl)phenoxy)ethoxy)ethyl)ammonium Chloride
55. Benzenemethanaminium, N,n-dimethyl-n-(2-(2-(4-(1,1,3,3-tetramethylbutyl)phenoxy)ethoxy)ethyl)-, Chloride (1:1)
56. Formula 144
57. Nsc20200
58. Caswell No. 614b
59. Sr-05000001572
60. Ncgc00016373-03
61. Ammonium, Benzyldimethyl(2-(2-(p-(1,1,3,3-tetramethylbutyl)phenoxy)ethoxy)ethyl)-, Chloride, Monohydrate
62. Cas-121-54-0
63. Mfcd00011742
64. Bzt (van)
65. Benzetonio Cloruro [dcit]
66. Salanine
67. Ccris 4748
68. Hsdb 567
69. [2-[2-(4-diisobutylphenoxy)ethoxy]ethyl]dimethylbenzylammonium Chloride
70. {2-[2-(4-diisobutylphenoxy)ethoxy]ethyl}dimethylbenzylammonium Chloride
71. Unii-ph41d05744
72. Antiseptol (quarternary Compound)
73. Phemersol Chloride
74. Benzyldimethyl(2-{2-[4-(1,1,3,3-tetramethylbutyl)phenoxy]ethoxy}ethyl)ammonium Chloride
75. Benzyldimethyl(2-{2-[p-(1,1,3,3-tetramethylbutyl)phenoxy]ethoxy}ethyl)ammonium Chloride
76. Microklenz (tn)
77. Benzethonii Chloridum [inn-latin]
78. Nci-c61494
79. Benzenemethanaminium, N,n-dimethyl-n-[2-[2-[4-(1,1,3,3-tetramethylbutyl)phenoxy]ethoxy]ethyl]-, Chloride (1:1)
80. N,n-dimethyl-n-(2-{2-[4-(1,1,3,3-tetramethylbutyl)phenoxy]ethoxy}ethyl)-benzenemethanaminium Chloride
81. N-benzyl-n,n-dimethyl-2-{2-[4-(2,4,4-trimethylpentan-2-yl)phenoxy]ethoxy}ethanaminium Chloride
82. Prestwick_995
83. Einecs 204-479-9
84. Hyamine (tn)
85. Cloruro De Benzetonio [inn-spanish]
86. Nsc 20200
87. Chlorure De Benzethonium [inn-french]
88. Epa Pesticide Chemical Code 069154
89. Neostelin Green (tn)
90. Benzethonium Chloride [usp:inn:ban:jan]
91. Ec 204-479-9
92. Benzethonium Chloride, 97%
93. Benzyldimethyl(2-{2-[4-(2,4,4-trimethylpentan-2-yl)phenoxy]ethoxy}ethyl)azanium Chloride
94. Unii-rs89wu8v92
95. Benzethonium Chloride Hydrate
96. Schembl21713
97. Mls002153968
98. Spectrum1500138
99. Dtxsid6023810
100. Hms502g17
101. Benzethonium Chloride [ii]
102. Benzethonium Chloride [mi]
103. Hms1570m17
104. Hms1920g07
105. Hms2091m09
106. Hms2097m17
107. Hms2230m22
108. Hms3373h09
109. Hms3652d03
110. Hms3714m17
111. Hms3885p07
112. Pharmakon1600-01500138
113. Benzethonium Chloride [inn]
114. Benzethonium Chloride [jan]
115. Diisobutyl Phenoxy Ethoxy Ethyl Dimethyl Benzyl Ammonium Chloride
116. Hy-b0942
117. Benzethonium Chloride [hsdb]
118. Benzethonium Chloride [inci]
119. Tox21_110403
120. Tox21_202488
121. Benzethonium Chloride [vandf]
122. Ccg-39713
123. Nsc755908
124. P-diisobutylphenoxyethoxyethyldimethylbenzylammonium Chloride Monohydrate
125. S4162
126. Benzethonium Chloride [mart.]
127. 2-(2-(p-(diisobutyl)phenoxy)ethoxy)ethyl Dimethyl Benzyl Ammonium Chloride
128. Akos005287417
129. Benzethonium Chloride [usp-rs]
130. Benzethonium Chloride [who-dd]
131. Tox21_110403_1
132. Nsc-755908
133. Ammonium, Benzyldimethyl(2-(2-(p-(1,1,3,3-tetramethylbutyl)phenoxy)ethoxy)ethyl)-, Chloride
134. Benzethonium Chloride (jp17/usp/inn)
135. Ncgc00016373-08
136. Ncgc00091528-01
137. Ncgc00091528-02
138. Ncgc00094597-01
139. Ncgc00094597-02
140. Ncgc00094597-03
141. Ncgc00094597-04
142. Ncgc00260037-01
143. As-14646
144. Smr001233307
145. Benzethonium Chloride [ep Impurity]
146. Benzethonium Chloride [ep Monograph]
147. Benzethonium Chloride [usp Impurity]
148. Db-053360
149. B0044
150. Ft-0622657
151. Ft-0635910
152. Hyamine(r) 1622 Solution, 4 Mm In H2o
153. Sw197077-3
154. A13653
155. Benzethonium Chloride, Tested According To Usp
156. D01140
157. N11901
158. Benzethonium Chloride 1000 Microg/ml In Methanol
159. Benzethonium Chloride, Bioultra, >=99.0% (at)
160. Q425119
161. J-200196
162. Sr-05000001572-1
163. Sr-05000001572-3
164. Benzethonium Chloride Concentrate [usp Monograph]
165. F2173-1223
166. Z1317839149
167. Benzethonium Chloride, >=97% (titration), >=98% (hplc)
168. [diisobutyl(phenoxyethoxy)ethyl]dimethylbenzylammonium Chloride
169. P-diisobutylphenoxyethoxyethyl Dimethyl Benzyl Ammonium Chloride
170. [[(diisobutyl)phenoxyethoxyethyl]dimethylbenzyl]ammonium Chloride
171. [[(diisobutylphenoxy)ethoxy]ethyl] Dimethyl Benzyl Ammonium Chloride
172. Ammonium,1,3,3-tetramethylbutyl)phenoxy]ethoxy]ethyl]-, Chloride
173. Ammonium,1,3,3-tetramethylbutyl)phenoxy]ethoxy]ethyl]-,chloride
174. Benzethonium Chloride, European Pharmacopoeia (ep) Reference Standard
175. Benzethonium Chloride, United States Pharmacopeia (usp) Reference Standard
176. Benzethonium Chloride, Pharmaceutical Secondary Standard; Certified Reference Material
177. Benzyldimethyl-[[[p-(1,3,3-tetramethylbutyl)phenoxy]ethoxy]ethyl]ammonium Chloride
178. Benzyldimethyl[2-[2-[4-(1,3,3-tetramethylbutyl)phenoxy]ethoxy]ethyl]ammonium Chloride
179. Benzyldimethyl[2-[2-[p-(1,3,3-tetramethylbutyl)phenoxy]ethoxy]ethyl]ammonium Chloride
180. Wln: 1x1 & 1 & 1x1 & 1 & R Do2o2k1 & 1 & 1r & Q & G
181. Benzenemethanaminium, N,n-dimethyl-n-(2-(2-(4-(1,1,3,3-tetramethylbutyl)phenoxy)ethoxy)- Ethyl)-, Chloride
182. Benzenemethanaminium, N,n-dimethyl-n-(2-(2-(4-(1,1,3,3-tetramethylbutyl)phenoxy)ethoxy)ethyl)-, Chloride, Monohydrate
183. Benzenemethanaminium,n-dimethyl-n-[2-[2-[4-(1,1,3,3-tetramethylbutyl)phenoxy]ethoxy]ethyl]-, Chloride
184. N,n-dimethyl-n-(2-(2-(4-(1,1,3,3-tetramethylbutyl)phenoxy)ethoxy)- Ethyl)benzenemethanaminium Chloride
185. N-benzyl-n,n-dimethyl-2-(2-(4-(2,4,4-trimethylpentan-2-yl)phenoxy)ethoxy)ethanaminiumchloride
| Molecular Weight | 448.1 g/mol |
|---|---|
| Molecular Formula | C27H42ClNO2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 12 |
| Exact Mass | 447.2904073 g/mol |
| Monoisotopic Mass | 447.2904073 g/mol |
| Topological Polar Surface Area | 18.5 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 466 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Anti-Infective Agents, Local
Substances used on humans and other animals that destroy harmful microorganisms or inhibit their activity. They are distinguished from DISINFECTANTS, which are used on inanimate objects. (See all compounds classified as Anti-Infective Agents, Local.)
D - Dermatologicals
D08 - Antiseptics and disinfectants
D08A - Antiseptics and disinfectants
D08AJ - Quaternary ammonium compounds
D08AJ08 - Benzethonium chloride
PERCUTANEOUS ABSORPTION IS PROBABLY INSIGNIFICANT. /BENZALKONIUM CHLORIDE/
Gosselin, R.E., R.P. Smith, H.C. Hodge. Clinical Toxicology of Commercial Products. 5th ed. Baltimore: Williams and Wilkins, 1984., p. II-280

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
CAS Number : 107-07-3
End Use API : Benzethonium Chloride
About The Company : Viswaat Chemicals Limited was incorporated in the year 1999. Our business model includes manufacture and sale of various specialty chemicals catering to industr...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Related Excipient Companies

Dosage Form : Tablet
Grade : Oral
Dosage Form : Cream / Lotion / Ointment
Grade : Topical
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Tablet
Grade : Oral
Application : Taste Masking
Excipient Details : CM90 is a directly compressible, granulated calcium carbonate with maltodextrin used for swallow tablets due to its high density and compressibility.
Pharmacopoeia Ref : NA
Technical Specs : PSD D50: 490- 500, Tapped Density: 1.50
Ingredient(s) : Calcium Carbonate Excipient
Dosage Form : Orodispersible Tablet
Grade : Oral
Application : Chewable & Orodispersible Aids
Excipient Details : CS90 is a directly compressible calcium carbonate with starch used for chewable tablets due to its smooth mouthfeel and creamy texture.
Pharmacopoeia Ref : NA
Technical Specs : PSD D50: 150-175, Tapped Density: 0.85
Ingredient(s) : Calcium Carbonate Excipient
Dosage Form : Orodispersible Tablet
Grade : Oral
Dosage Form : Tablet
Grade : Oral
Application : Direct Compression
Excipient Details : DC SIM 100 is a directly compressible simethicone powder used for antacid and anti-gas tablets.
Dosage Form : Tablet
Grade : Oral
Dosage Form : Orodispersible Tablet
Grade : Oral
Application : Chewable & Orodispersible Aids
Excipient Details : Vitasmooth is a directly compressible calcium carbonate, preformulated with excipients used for the production of chewable or antacid meltaway tablets
Pharmacopoeia Ref : NA
Technical Specs : Tapped Density: 0.80, PSD D50: 153
Ingredient(s) : Calcium Carbonate Excipient
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Gel
Grade : Parenteral, Oral, Topical
Dosage Form : Injectable / Parenteral
Grade : Parenteral
Application : Parenteral
Excipient Details : Castor oil is used as an oily solvent in parenteral dosage forms.
Dosage Form : Cream / Lotion / Ointment
Grade : Topical
Application : Topical
Excipient Details : Octyldodecanol is used as a solvent in topical dosage forms.
Dosage Form : Injectable / Parenteral
Grade : Parenteral, Oral, Topical
Application : Solubilizers
Excipient Details : Poloxamer 188 is used as a solubilizer, emulsifier, stabilizer in parenteral, OSDs & topical formulations such as creams, gels & lotions.
Pharmacopoeia Ref : ChP/USP/EP
Technical Specs : Oxyethylene unit: 75%-85%, Oxypropylene unit: 25%-30%
Ingredient(s) : Poloxamer 188
Dosage Form : Injectable / Parenteral
Grade : Parenteral, Topical, Oral
Dosage Form : Cream / Lotion / Ointment
Grade : Topical
Dosage Form : Cream / Lotion / Ointment
Grade : Parenteral, Oral, Topical
Dosage Form : Cream / Lotion / Ointment
Grade : Topical, Oral
Application : Solubilizers
Excipient Details : Polysorbate 40 is used as a wetting and solubilizing agent in various topical and oral formulations.
Dosage Form : Injectable / Parenteral
Grade : Parenteral, Oral, Topical
Dosage Form : Injectable / Parenteral
Grade : Parenteral, Oral, Topical
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Tablet
Grade : Oral
Application : Thickeners and Stabilizers
Excipient Details : Adsorbent, Moisture Protection, Stabilization of API
Pharmacopoeia Ref : USP-NF, JP, EP
Technical Specs : Also Available as FUJISIL-F
Ingredient(s) : Silicon Dioxide
Dosage Form : Cream / Lotion / Ointment
Grade : Oral, Parenteral, Topical
Brand Name : MONTANE 60 PHA PREMIUM
Application : Emulsifying Agents
Pharmacopoeia Ref : USP-NF, EP, JP
Technical Specs : Non-Ionic Lipophilic surfactant; Emulsifier (w/o emulsions)
Ingredient(s) : Sorbitan Monostearate
Dosage Form : Tablet
Grade : Oral, Parenteral, Topical
Brand Name : MONTANE 80 PHA PREMIUM
Application : Emulsifying Agents
Pharmacopoeia Ref : USP-NF, EP, JP
Technical Specs : Non-Ionic Lipophilic surfactant; Emulsifier (w/o emulsions)
Ingredient(s) : sorbitan oleate
Dosage Form : Tablet
Grade : Oral, Topical
Brand Name : MONTANOX 80 PHA PREMIUM
Application : Emulsifying Agents
Pharmacopoeia Ref : USP-NF, EP, JP
Technical Specs : Non-Ionic Hydrophilic surfactant, Emulsifier (o/w emulsion), Solubilizer
Ingredient(s) : Polysorbate 80
Dosage Form : Capsule
Grade : Oral
Application : Coating Systems & Additives
Excipient Details : Ready mix Film coating system for moisture sensitive APIs
Pharmacopoeia Ref : USP, EP, JP; Having US-DMF
Technical Specs : Moisture barrier film coating system
Ingredient(s) : Hydroxypropyl Methyl Cellulose
Dosage Form : Capsule
Grade : Oral
Application : Coating Systems & Additives
Excipient Details : Ready mix Non-Functional film coating system.
Pharmacopoeia Ref : USP, EP, JP; Having US-DMF
Technical Specs : Not Available
Ingredient(s) : Hydroxypropyl Methyl Cellulose
Dosage Form : Capsule
Grade : Oral
Application : Taste Masking
Excipient Details : Ready mix sugar coating system.
Pharmacopoeia Ref : USP, EP, JP & having US DMF
Technical Specs : Sprayable sugar coating system for solid oral dosage form
Ingredient(s) : Hydroxypropyl Methyl Cellulose
Dosage Form : Emulsion
Grade : Topical
Application : Rheology Modifiers
Excipient Details : Thickener, Emulsifier, Stabilizer, Texturizing agent, pH Independent & Non Thixotropic polymer for Topical Range (Skin,Vaginal & Anal mucosa)
Pharmacopoeia Ref : In house having US DMF Type IV...
Technical Specs : Ready to use liquid polymer for topical applications (Gel / Cream / Lotion/ Foam based formulation, ...
Ingredient(s) : Hydroxyethyl Acrylate
Dosage Form : Capsule
Grade : Oral
Application : Solubilizers
Excipient Details : Polyoxyl 40 hydrogenated castor oil is used as solubilizer and emulsifying agent.
Pharmacopoeia Ref : USP-NF, EP, JP
Technical Specs : Solubilizer in powder form, used in Directly compressible dosage forms, Wet granulation, added durin...
Ingredient(s) : Magnesium Aluminometasilicate
Dosage Form : Capsule
Grade : Oral
Application : Solubilizers
Excipient Details : Polysorbate 80 in dry powder form, a solubilizing agent acts as a surfactant and increases the solubility of various oral dosage forms.
Pharmacopoeia Ref : USP-NF, EP, JP & having US DMF
Technical Specs : Solubilizer in powder form, used in directly compressible dosage forms, Wet granulation, added durin...
Ingredient(s) : Magnesium aluminium silicate Excipient
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Excipients by Applications
Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
A Hyamine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Hyamine, including repackagers and relabelers. The FDA regulates Hyamine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Hyamine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Hyamine manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Hyamine supplier is an individual or a company that provides Hyamine active pharmaceutical ingredient (API) or Hyamine finished formulations upon request. The Hyamine suppliers may include Hyamine API manufacturers, exporters, distributors and traders.
click here to find a list of Hyamine suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Hyamine Drug Master File in Japan (Hyamine JDMF) empowers Hyamine API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Hyamine JDMF during the approval evaluation for pharmaceutical products. At the time of Hyamine JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Hyamine suppliers with JDMF on PharmaCompass.
A Hyamine written confirmation (Hyamine WC) is an official document issued by a regulatory agency to a Hyamine manufacturer, verifying that the manufacturing facility of a Hyamine active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Hyamine APIs or Hyamine finished pharmaceutical products to another nation, regulatory agencies frequently require a Hyamine WC (written confirmation) as part of the regulatory process.
click here to find a list of Hyamine suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Hyamine as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Hyamine API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Hyamine as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Hyamine and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Hyamine NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Hyamine suppliers with NDC on PharmaCompass.
Hyamine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Hyamine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Hyamine GMP manufacturer or Hyamine GMP API supplier for your needs.
A Hyamine CoA (Certificate of Analysis) is a formal document that attests to Hyamine's compliance with Hyamine specifications and serves as a tool for batch-level quality control.
Hyamine CoA mostly includes findings from lab analyses of a specific batch. For each Hyamine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Hyamine may be tested according to a variety of international standards, such as European Pharmacopoeia (Hyamine EP), Hyamine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Hyamine USP).


LOOKING FOR A SUPPLIER?