
Synopsis
Synopsis
0
KDMF
0
VMF
0
Canada

0
Australia

0
South Africa

0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
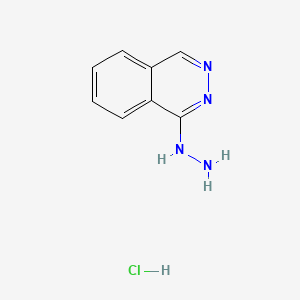

1. Apresoline
2. Apressin
3. Apressoline
4. Hydralazine
5. Hydralazine Mono Hydrochloride
6. Hydralazine Mono-hydrochloride
7. Hydrallazin
8. Hydrazinophthalazine
9. Hydrochloride, Hydralazine
10. Mono-hydrochloride, Hydralazine
11. Nepresol
1. 304-20-1
2. 1-hydrazinophthalazine Hydrochloride
3. Hydralazine Hcl
4. Apresoline
5. 1-hydrazinylphthalazine Hydrochloride
6. Aiselazine
7. Dralzine
8. Hydralazine Chloride
9. Hyperazin
10. Hyperex
11. Lopress
12. Slow-apresolin
13. Hydralazine Monohydrochloride
14. Apresoline Hydrochloride
15. 1-hydrazinophthalazine Monohydrochloride
16. Hydralazine (hydrochloride)
17. 1(2h)-phthalazinone, Hydrazone, Monohydrochloride
18. Phthalazin-1-ylhydrazine;hydrochloride
19. Praparat 5968
20. Nsc-89394
21. Phthalazine, 1-hydrazino-, Monohydrochloride
22. Chebi:31672
23. Fd171b778y
24. Mc-1101
25. Apresine
26. Rolazine
27. Apresoline-esidrix
28. Dsstox_cid_24645
29. Dsstox_rid_80373
30. Dsstox_gsid_44645
31. Phthalazine, 1-hydrazinyl-, Hydrochloride (1:1)
32. 1044569-46-1
33. Nor-press 25
34. Hydrallazine Hydrochloride
35. Ccris 334
36. Hsdb 434
37. Hydralazine Hydrochloride [jan]
38. Sr-01000075903
39. Ncgc00015501-02
40. 1(2h)-phthalazinone, Hydrazone, Hydrochloride
41. Cas-304-20-1
42. Einecs 206-151-0
43. Nsc 89394
44. Apressinum
45. Phthalazinone, Hydrazone, Monohydrochloride
46. Unii-fd171b778y
47. Apresoline (tn)
48. Prestwick_610
49. Hydralazine Hydrochloride [usp:jan]
50. Hydralazinhydrochlorid
51. Mfcd00135998
52. 1-hydrazinyl-phthalazine Hydrochloride
53. Ophthazin Impurity 2
54. Hydralazinehydrochloride
55. Hydralazini Hydrochloridum
56. 1-hydrazinophthalazine Hcl
57. Apressinum [who-ip]
58. Schembl36271
59. Phthalazine, Monohydrochloride
60. Spectrum1500334
61. Chembl542541
62. Phthalazinone, Monohydrochloride
63. Dtxsid1044645
64. Bpi-103
65. Pra 027
66. Hms1568b19
67. Hms1920d17
68. Pharmakon1600-01500334
69. Hy-b0464
70. Nsc89394
71. Wln: T66 Cnnj Bmz & Gh
72. 1(2h)-phthalazinone, Hydrochloride
73. Tox21_110163
74. Tox21_302496
75. Tox21_500593
76. Ccg-40185
77. Nsc757058
78. S2562
79. 1-hydrazinophthalazinemonohydrochloride
80. Hydralazine Hydrochloride [mi]
81. Akos008968535
82. Akos025116605
83. Akos028109609
84. Hydralazine Hydrochloride (jp17/usp)
85. Tox21_110163_1
86. 1(2h)-phthalazinone, Monohydrochloride
87. Ccg-266561
88. Lp00593
89. Nsc-757058
90. Hydralazine Hydrochloride [hsdb]
91. Phthalazinone Hydrazone Monohydrochloride
92. Hydralazine Hydrochloride [mart.]
93. Hydralazine Hydrochloride [vandf]
94. Ncgc00015501-09
95. Ncgc00093972-01
96. Ncgc00093972-02
97. Ncgc00093972-03
98. Ncgc00093972-04
99. Ncgc00256719-01
100. Ncgc00261278-01
101. Ac-18050
102. Hydralazine Hydrochloride [usp-rs]
103. Hydralazine Hydrochloride [who-dd]
104. Hydralazine Hydrochloride [who-ip]
105. 1(2h)-phthalazinone Hydrazone Hydrochloride
106. Eu-0100593
107. Ft-0669283
108. H0409
109. En300-03637
110. Bidil Component Hydralazine Hydrochloride
111. D01302
112. H 1753
113. Hydralazine Hydrochloride [orange Book]
114. Hydralazine Hydrochloride [usp Impurity]
115. Hydralazine Hydrochloride [usp Monograph]
116. Hydralazini Hydrochloridum [who-ip Latin]
117. A820366
118. Dralserp Component Hydralazine Hydrochloride
119. Hydralazine Hydrochloride Component Of Bidil
120. Unipres Component Hydralazine Hydrochloride
121. Apresazide Component Hydralazine Hydrochloride
122. Cam-ap-es Component Hydralazine Hydrochloride
123. Hydrap-es Component Hydralazine Hydrochloride
124. Q-201208
125. Ser-a-gen Component Hydralazine Hydrochloride
126. Ser-ap-es Component Hydralazine Hydrochloride
127. Sr-01000075903-1
128. Sr-01000075903-5
129. (z)-1-hydrazono-1,2-dihydrophthalazine Hydrochloride
130. Hydra-zide Component Hydralazine Hydrochloride
131. Hydralazine Hydrochloride Component Of Dralserp
132. Hydralazine Hydrochloride Component Of Unipres
133. Q27114606
134. Z57980178
135. Hydralazine Hydrochloride Component Of Apresazide
136. Hydralazine Hydrochloride Component Of Cam-ap-es
137. Hydralazine Hydrochloride Component Of Hydra-zide
138. Hydralazine Hydrochloride Component Of Hydrap-es
139. Hydralazine Hydrochloride Component Of Ser-a-gen
140. Hydralazine Hydrochloride Component Of Ser-ap-es
141. Apresoline-esidrix Component Hydralazine Hydrochloride
142. Hydralazine Hydrochloride Component Of Apresoline-esidrix
143. Serpasil-apresoline Component Hydralazine Hydrochloride
144. Hydralazine Hydrochloride Component Of Serpasil-apresoline
145. Hydroserpine Plus (r-h-h) Component Hydralazine Hydrochloride
146. Hydralazine Hydrochloride Component Of Hydroserpine Plus (r-h-h)
147. Hydralazine Hydrochloride Component Of Hydralazine Hydrochloride-hydrochlorothiazide-reserpine
148. Hydralazine Hydrochloride-hydrochlorothiazide-reserpine Component Hydralazine Hydrochloride
| Molecular Weight | 196.64 g/mol |
|---|---|
| Molecular Formula | C8H9ClN4 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 1 |
| Exact Mass | 196.0515740 g/mol |
| Monoisotopic Mass | 196.0515740 g/mol |
| Topological Polar Surface Area | 63.8 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 150 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Hydralazine hydrochloride |
| Drug Label | HydrALAZINE hydrochloride, USP, is an antihypertensive, for oral administration. Its chemical name is 1-hydrazinophthalazine monohydrochloride, and its structural formula is:C8H8N4HClHydrALAZINE hydrochloride, USP is a white to off-white, odorless cr... |
| Active Ingredient | Hydralazine hydrochloride |
| Dosage Form | Tablet; Injectable |
| Route | Injection; Oral |
| Strength | 20mg/ml; 25mg; 100mg; 50mg; 10mg |
| Market Status | Prescription |
| Company | Navinta; Glenmark Pharms; Cadila Pharms; Alkem Labs; Hetero Labs Ltd Iii; Invagen Pharms; Par Pharm; Actavis Grp Ptc; Strides Pharma; Fresenius Kabi Usa; Luitpold; Pliva; Mylan; Heritage Pharms; Akorn |
| 2 of 2 | |
|---|---|
| Drug Name | Hydralazine hydrochloride |
| Drug Label | HydrALAZINE hydrochloride, USP, is an antihypertensive, for oral administration. Its chemical name is 1-hydrazinophthalazine monohydrochloride, and its structural formula is:C8H8N4HClHydrALAZINE hydrochloride, USP is a white to off-white, odorless cr... |
| Active Ingredient | Hydralazine hydrochloride |
| Dosage Form | Tablet; Injectable |
| Route | Injection; Oral |
| Strength | 20mg/ml; 25mg; 100mg; 50mg; 10mg |
| Market Status | Prescription |
| Company | Navinta; Glenmark Pharms; Cadila Pharms; Alkem Labs; Hetero Labs Ltd Iii; Invagen Pharms; Par Pharm; Actavis Grp Ptc; Strides Pharma; Fresenius Kabi Usa; Luitpold; Pliva; Mylan; Heritage Pharms; Akorn |
Antihypertensive Agents; Vasodilator Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
MEDICATION (VET): Use in exptl medicine and surgery. Use in dogs with hydrocortisone in hemorrhagic Shock or endotoxic shock markedly promoted survival. Increases renal blood flow.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 263
Hydralazine is indicated orally in the treatment of hypertension. Hydralazine is indicated intravenously when oral therapy cannot be given or when there is an urgent need to lower blood pressure, such as in hypertensive crisis or pre-eclampsia or eclampsia. /Included in US product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1661
Hydralazine combined with isosorbide dinitrate (nonspecific vasodilator therapy) has been used as a supplement to the traditional congestive heart failure treatment of digitalis and diuretics. /NOT included in US or Canadian product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1661
For more Therapeutic Uses (Complete) data for HYDRALAZINE HYDROCHLORIDE (6 total), please visit the HSDB record page.
Following parenteral administration to patients with coronary artery disease, the myocardial ischemia may be sufficiently severe and protracted to cause frank myocardial infarction. For this reason, parenteral administration of hydralazine is contraindicated in hypertensive patients with coronary artery disease and inadvisable for most hypertensive patients over 40 years old. In addition, if the drug is used alone, there may be salt retention with development of high-output congestive heart failure.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 886
VET: Chronic use may cause various collagen diseases & a lupus erythematosus syndrome (rats, dogs).
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 263
Maternal Medication usually Compatible with Breast-Feeding: Hydralazine: Reported Sign or Symptom in Infant or Effect on Lactation: None. /from Table 6/
Report of the American Academy of Pediatrics Committee on Drugs in Pediatrics 93 (1): 141 (1994)
Use of hydralazine alone /for congestive heart failure/ is not recommended, although it may improve cardiac performance in some patients with intractable left ventricular failure.
USP Convention. USPDI - Drug Information for the Health Care Professional. 16th ed. Volume I. Rockville, MD: U.S. Pharmaceutical Convention, Inc. 1996 (Plus updates)., p. 1629
For more Drug Warnings (Complete) data for HYDRALAZINE HYDROCHLORIDE (18 total), please visit the HSDB record page.
Highest known dose survived: adults, 10 g orally.
Medical Economics Co; Physicians Desk Reference Generics 2nd ed p. 1553 (1996)
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
Vasodilator Agents
Drugs used to cause dilation of the blood vessels. (See all compounds classified as Vasodilator Agents.)
Hydralazine is well absorbed through the GI tract, but the systemic bioavailability is low (16% in fast acetylators and 35% in slow acetylators).
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 886
The peak concentration of hydralazine in plasma and the peak hypotensive effect of the drug occur within 30 to 120 minutes of ingestion. Although its half-life in plasma is about an hour, the duration of the hypotensive effect of hydralazine can last as long as 12 hours. There is no clear explanation for this discrepancy.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 886
Compound /3-methyltriazolo(5,1-b)phthalazine/ is one of the major metabolites of hydralazine in human subjects.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 2: A Review of the Literature Published Between 1970 and 1971. London: The Chemical Society, 1972., p. 180
Hydralazine is N-acetylated in the bowel and/or the liver. The rate of acetylation is genetically determined; about half of the people in the United States acetylate rapidly and half do so slowly.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 886
Since the systemic clearance exceeds hepatic blood flow, extrahepatic metabolism must occur. ... Hydralazine rapidly combines with circulating alpha-keto acids to form hydrazones, and the major metabolite recovered from the plasma is hydralazine pyruvic acid hydrazone.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 886
... Pharmacokinetic data indicate hydralazine ... has an extensive and complex metabolism depending on acetylator status: slow acetylators undergo primary oxidative metabolism, while rapid acetylators are acetylated. ...
PMID:2656046 Mulrow JP, Crawford MH; Clin Pharmacokinet 16 (2): 86-9 (1989)
The half-life of hydralazine is 1 hour, and the systemic clearance of the drug is about 50 ml/kg per minute.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 886
Hydralazine causes direct relaxation of arteriolar smooth muscle. The molecular mechanism of this effect is not known. It is not a dilator of capacitance vessels (e.g., the epicardial coronary arteries) and does not relax venous smooth muscle. Hydralazine-induced vasodilatation is associated with powerful stimulation of the sympathetic nervous system, which results in increased heart rate and contractility, increased plasma renin activity, and fluid retention; all of these effects counteract the antihypertensive effect of hydralazine. Although most of the sympathetic activity is due to a baroreceptor-mediated reflex, hydralazine may stimulate the release of norepinephrine from sympathetic nerve terminals and augment myocardial contractility directly.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 885

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
51
PharmaCompass offers a list of Hydralazine Hydrochloride API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Hydralazine Hydrochloride manufacturer or Hydralazine Hydrochloride supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Hydralazine Hydrochloride manufacturer or Hydralazine Hydrochloride supplier.
PharmaCompass also assists you with knowing the Hydralazine Hydrochloride API Price utilized in the formulation of products. Hydralazine Hydrochloride API Price is not always fixed or binding as the Hydralazine Hydrochloride Price is obtained through a variety of data sources. The Hydralazine Hydrochloride Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Hydralazine Hydrochloride manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Hydralazine Hydrochloride, including repackagers and relabelers. The FDA regulates Hydralazine Hydrochloride manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Hydralazine Hydrochloride API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Hydralazine Hydrochloride manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Hydralazine Hydrochloride supplier is an individual or a company that provides Hydralazine Hydrochloride active pharmaceutical ingredient (API) or Hydralazine Hydrochloride finished formulations upon request. The Hydralazine Hydrochloride suppliers may include Hydralazine Hydrochloride API manufacturers, exporters, distributors and traders.
click here to find a list of Hydralazine Hydrochloride suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Hydralazine Hydrochloride DMF (Drug Master File) is a document detailing the whole manufacturing process of Hydralazine Hydrochloride active pharmaceutical ingredient (API) in detail. Different forms of Hydralazine Hydrochloride DMFs exist exist since differing nations have different regulations, such as Hydralazine Hydrochloride USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Hydralazine Hydrochloride DMF submitted to regulatory agencies in the US is known as a USDMF. Hydralazine Hydrochloride USDMF includes data on Hydralazine Hydrochloride's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Hydralazine Hydrochloride USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Hydralazine Hydrochloride suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Hydralazine Hydrochloride Drug Master File in Japan (Hydralazine Hydrochloride JDMF) empowers Hydralazine Hydrochloride API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Hydralazine Hydrochloride JDMF during the approval evaluation for pharmaceutical products. At the time of Hydralazine Hydrochloride JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Hydralazine Hydrochloride suppliers with JDMF on PharmaCompass.
A Hydralazine Hydrochloride CEP of the European Pharmacopoeia monograph is often referred to as a Hydralazine Hydrochloride Certificate of Suitability (COS). The purpose of a Hydralazine Hydrochloride CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Hydralazine Hydrochloride EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Hydralazine Hydrochloride to their clients by showing that a Hydralazine Hydrochloride CEP has been issued for it. The manufacturer submits a Hydralazine Hydrochloride CEP (COS) as part of the market authorization procedure, and it takes on the role of a Hydralazine Hydrochloride CEP holder for the record. Additionally, the data presented in the Hydralazine Hydrochloride CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Hydralazine Hydrochloride DMF.
A Hydralazine Hydrochloride CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Hydralazine Hydrochloride CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Hydralazine Hydrochloride suppliers with CEP (COS) on PharmaCompass.
A Hydralazine Hydrochloride written confirmation (Hydralazine Hydrochloride WC) is an official document issued by a regulatory agency to a Hydralazine Hydrochloride manufacturer, verifying that the manufacturing facility of a Hydralazine Hydrochloride active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Hydralazine Hydrochloride APIs or Hydralazine Hydrochloride finished pharmaceutical products to another nation, regulatory agencies frequently require a Hydralazine Hydrochloride WC (written confirmation) as part of the regulatory process.
click here to find a list of Hydralazine Hydrochloride suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Hydralazine Hydrochloride as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Hydralazine Hydrochloride API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Hydralazine Hydrochloride as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Hydralazine Hydrochloride and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Hydralazine Hydrochloride NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Hydralazine Hydrochloride suppliers with NDC on PharmaCompass.
Hydralazine Hydrochloride Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Hydralazine Hydrochloride GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Hydralazine Hydrochloride GMP manufacturer or Hydralazine Hydrochloride GMP API supplier for your needs.
A Hydralazine Hydrochloride CoA (Certificate of Analysis) is a formal document that attests to Hydralazine Hydrochloride's compliance with Hydralazine Hydrochloride specifications and serves as a tool for batch-level quality control.
Hydralazine Hydrochloride CoA mostly includes findings from lab analyses of a specific batch. For each Hydralazine Hydrochloride CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Hydralazine Hydrochloride may be tested according to a variety of international standards, such as European Pharmacopoeia (Hydralazine Hydrochloride EP), Hydralazine Hydrochloride JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Hydralazine Hydrochloride USP).
