
Synopsis
0
VMF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Dichlothiazide
2. Dihydrochlorothiazide
3. Esidrex
4. Esidrix
5. Hctz
6. Hydrodiuril
7. Hypothiazide
8. Oretic
9. Sectrazide
1. 58-93-5
2. Hctz
3. Esidrix
4. Hypothiazide
5. Hidrotiazida
6. Idrotiazide
7. Megadiuril
8. Newtolide
9. Servithiazid
10. Oretic
11. Vetidrex
12. Chlorosulthiadil
13. Esidrex
14. Dichlotiazid
15. Hydrochlorothiazid
16. Hydrochlorthiazide
17. Hydrodiuretic
18. Hydrosaluric
19. Apresazide
20. Aquarills
21. Aquarius
22. Carozide
23. Dichlotride
24. Diclotride
25. Disalunil
26. Hypothiazid
27. Maschitt
28. Thiuretic
29. Drenol
30. Hidril
31. Hydril
32. Nefrix
33. Hidrochlortiazid
34. Hydrodiuril
35. Hydro-diuril
36. Hydro-aquil
37. Dihydrochlorothiazide
38. Jen-diril
39. Dihydrochlorothiazid
40. Lotensin Hct
41. Dihydrochlorothiazidum
42. Chlorzide
43. Dichlorosal
44. Hidroronol
45. Hydrothide
46. Hydrozide
47. Microzide
48. Moduretic
49. Neoflumen
50. Urodiazin
51. Bremil
52. Cidrex
53. Diaqua
54. Direma
55. Dyazide
56. Fluvin
57. Ivaugan
58. Panurin
59. Ro-hydrazide
60. Neo-codema
61. Acuretic
62. Caplaril
63. Hydrocot
64. Pantemon
65. Thlaretic
66. Timolide
67. Unipres
68. Ziac
69. Zide
70. 6-chloro-3,4-dihydro-2h-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide
71. Dihydrochlorurite
72. Aquazide H
73. Hydro-d
74. 3,4-dihydrochlorothiazide
75. Hydrochlorothiazidum
76. Thiaretic
77. Vaseretic
78. Apo-hydro
79. Lopressor Hct
80. Inderide
81. Dihydrochlorurit
82. Hidroclorotiazida
83. Su 5879
84. Aldactazide
85. Maxzide
86. Prinzide
87. Hydrochlorothiazide Intensol
88. Nci-c55925
89. Component Of Cyclex
90. Component Of Esimil
91. Esidrix (tn)
92. Ser-ap-es
93. Component Of Aldoril
94. Component Of Dyazide
95. Component Of Caplaril
96. Hcz
97. Chlorsulfonamidodihydrobenzothiadiazine Dioxide
98. Component Of Aldactazide
99. Copalia-hct
100. Exforge-hct
101. Imprida-hct
102. Rasilez-hct
103. Dafiro-hct
104. Hydrochlorothiaizide
105. Component Of Butizide Prestabs
106. 2h-1,2,4-benzothiadiazine-7-sulfonamide, 6-chloro-3,4-dihydro-, 1,1-dioxide
107. 6-chloro-1,1-dioxo-3,4-dihydro-2h-1$l^{6},2,4-benzothiadiazine-7-sulfonamide
108. Nsc-53477
109. Diu-melusin
110. 0j48lph2th
111. 6-chloro-7-sulfamoyl-3,4-dihydro-2h-1,2,4-benzothiadiazine 1,1-dioxide
112. 6-chloro-3,4-dihydro-(2h)-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide
113. Mls000069619
114. Chebi:5778
115. Chlorizide
116. Disothiazid
117. Hct
118. Hidrosaluretil
119. Novodiurex
120. Spironazide
121. Acesistem
122. Acuilix
123. Aldazida
124. Briazide
125. Catiazida
126. Chlothia
127. Clorana
128. Condiuren
129. Dihydran
130. Diurogen
131. Dixidrasi
132. Esoidrina
133. H.h. 25/25
134. H.h. 50/50
135. Hyclosid
136. Indroclor
137. Manuril
138. Medozide
139. Mictrin
140. Mikorten
141. Modurcen
142. Natrinax
143. Saldiuril
144. Selozide
145. Tandiur
146. Urozide
147. Didral
148. Hytrid
149. Nefrol
150. Unazid
151. Hidro-niagrin
152. Hydro-saluric
153. Raunova Plus
154. 3,4-dihydro-6-chloro-7-sulfamyl-1,2,4-benzothiadiazine-1,1-dioxide
155. Concor Plus
156. Aquazide-h
157. Neo-flumen
158. Neo-minzil
159. 6-chloro-3,4-dihydro-7-sulfamoyl-2h-1,2,4-benzothiadiazine 1,1-dioxide
160. Hydro Par
161. Nsc53477
162. 6-chloro-1,1-dioxo-3,4-dihydro-2h-1lambda6,2,4-benzothiadiazine-7-sulfonamide
163. 6-chloro-3,4-dihydro-2h-benzo[e][1,2,4]thiadiazine-7-sulfonamide 1,1-dioxide
164. Hydro-t
165. Hydrochlorthiazidum
166. Hct-isis
167. Su-5879
168. Cas-58-93-5
169. Mazide 25 Mg
170. Ncgc00015508-08
171. Hydrochlorat
172. Hydrochlorot
173. Smr000035778
174. Idroclorotiazide
175. Diu 25 Vigt
176. Hydrex-semi
177. Aldactazide 25/25
178. Aldectazide 50/50
179. Dihydroxychlorothiazidum
180. Dsstox_cid_713
181. Inderide 80/25
182. Hydrochlorothiazide Form Ii
183. 6-chloro-3,4-dihydro-2h-1,2,4-benzothiadiazine-7-sulfonamide-1,1-dioxide
184. Idroclorotiazide [dcit]
185. Dsstox_rid_75752
186. Dsstox_gsid_20713
187. Hydrochlorzide
188. Ezide
189. Hydrozide Injection, Veterinary
190. Hidroclorotiazida [inn-spanish]
191. Hydrochlorothiazidum [inn-latin]
192. 6-chloro-1,1-dioxo-3,4-dihydro-2h-1,2,4-benzothiadiazine-7-sulfonamide
193. Microzide (tn)
194. Ccris 2082
195. Hsdb 3096
196. Sr-01000000119
197. Einecs 200-403-3
198. Nsc 53477
199. Unii-0j48lph2th
200. Brn 0625101
201. Hydrokraft
202. Idrodiuvis
203. Aquazide
204. Manschitt
205. Urirex
206. Hydropar
207. 6-chloro-1,1-dioxo-3,4-dihydro-2h-1?^{6},2,4-benzothiadiazine-7-sulfonamide
208. 6-chloro-7-sulfamyl-3,4-dihydro-1,2,4-benzothiadiazine 1,1-dioxide
209. Prestwick_263
210. Hydrochloro Thiazide
211. Hyzaar (salt/mix)
212. Mfcd00051765
213. Aldoril (salt/mix)
214. Dutoprol (salt/mix)
215. Inderide (salt/mix)
216. Prinzide (salt/mix)
217. Accuretic (salt/mix)
218. Hydropres (salt/mix)
219. Thiazide, Hydrochloro-
220. Hydrochlorothiazide [usp:inn:ban:jan]
221. Spectrum_000877
222. Opera_id_168
223. Maybridge1_004336
224. Prestwick0_000009
225. Prestwick1_000009
226. Prestwick2_000009
227. Prestwick3_000009
228. Spectrum2_001040
229. Spectrum3_000456
230. Spectrum4_000006
231. Spectrum5_000824
232. Lopac-h-4759
233. Chembl435
234. Tekturna Hct (salt/mix)
235. 2h-1,2,4-benzothiadiazine-7-sulfonamide, 6-chloro-3,4-dihydro-, 1, 1-dioxide
236. H 4759
237. Cid_3639
238. Schembl9349
239. Lopac0_000614
240. Oprea1_357174
241. Bspbio_000017
242. Bspbio_002132
243. Kbiogr_000351
244. Kbioss_001357
245. Af-614/30832002
246. Bidd:gt0153
247. Divk1c_000289
248. Spectrum1500335
249. Bmcl182567 Compound 6a
250. Spbio_001259
251. Spbio_001938
252. Bpbio1_000019
253. Gtpl4836
254. Hydrochlorothiazide, Crystalline
255. Hydrochlorothiazide [mi]
256. Dtxsid2020713
257. Bdbm13076
258. Component Of Dyazide (salt/mix)
259. Hms500o11
260. Hms553n04
261. Hydrochlorothiazide [inn]
262. Hydrochlorothiazide [jan]
263. Kbio1_000289
264. Kbio2_001357
265. Kbio2_003925
266. Kbio2_006493
267. Kbio3_001352
268. Hydrochlorothiazide [hsdb]
269. Hydrochlorothiazide [iarc]
270. Component Of Caplaril (salt/mix)
271. Ninds_000289
272. Hms1568a19
273. Hms1920d19
274. Hms2091l05
275. Hms2095a19
276. Hms2235i09
277. Hms3259o17
278. Hms3261l10
279. Hms3370p11
280. Hms3428a05
281. Hms3655m21
282. Hms3712a19
283. Hydrochlorothiazide [vandf]
284. Pharmakon1600-01500335
285. Zinc896569
286. Hydrochlorothiazide [mart.]
287. Hydrochlorothiazide Component
288. Bcp22001
289. Hy-b0252
290. Jfd00715
291. Hydrochlorothiazide [usp-rs]
292. Hydrochlorothiazide [who-dd]
293. Hydrochlorothiazide [who-ip]
294. Tox21_110165
295. Tox21_201565
296. Tox21_300292
297. Tox21_500614
298. 3,4-dihydro-6-chloro-7-sulfamoyl-1,2,4-benzothiadiazine-1,1-dioxide
299. Ccg-40240
300. Nsc757059
301. S1708
302. Stk315354
303. 3,2,4-benzothiadiazine-1,1-dioxide
304. Hydrochlorothiazide (jp17/usp/inn)
305. Hydrochlorothiazide [ema Epar]
306. Akos000121373
307. Tox21_110165_1
308. Ac-8114
309. Db00999
310. Lp00614
311. Nc00510
312. Nsc-757059
313. Ps-3162
314. Sdccgsbi-0050596.p005
315. Hydrochlorothiazide [green Book]
316. Idi1_000289
317. Wln: T66 Bswm Em Dhj Hg Iszw
318. Hydrochlorothiazide [ep Impurity]
319. Hydrochlorothiazide [orange Book]
320. Ncgc00015508-01
321. Ncgc00015508-02
322. Ncgc00015508-03
323. Ncgc00015508-04
324. Ncgc00015508-05
325. Ncgc00015508-06
326. Ncgc00015508-07
327. Ncgc00015508-09
328. Ncgc00015508-10
329. Ncgc00015508-11
330. Ncgc00015508-12
331. Ncgc00015508-13
332. Ncgc00015508-14
333. Ncgc00015508-16
334. Ncgc00015508-17
335. Ncgc00015508-25
336. Ncgc00021906-03
337. Ncgc00021906-04
338. Ncgc00021906-05
339. Ncgc00021906-06
340. Ncgc00021906-07
341. Ncgc00021906-08
342. Ncgc00254017-01
343. Ncgc00259114-01
344. Ncgc00261299-01
345. Ziac Component Hydrochlorothiazide
346. 8049-49-8
347. Bh164522
348. Hh-50/50
349. Hydrochlorothiazide [ep Monograph]
350. Nci60_004317
351. Component Of Butizide Prestabs (salt/mix)
352. Esimil Component Hydrochlorothiazide
353. Hydrochlorothiazide [usp Monograph]
354. Hydrochlorothiazide 1.0 Mg/ml In Methanol
355. Hydrochlorothiazidum [who-ip Latin]
356. Hyzaar Component Hydrochlorothiazide
357. Sbi-0050596.p004
358. Aldoril Component Hydrochlorothiazide
359. Avalide Component Hydrochlorothiazide
360. Dyazide Component Hydrochlorothiazide
361. Hydrochlorothiazide Component Of Ziac
362. Maxzide Component Hydrochlorothiazide
363. Polycap Component Hydrochlorothiazide
364. Riprazo Component Hydrochlorothiazide
365. Unipres Component Hydrochlorothiazide
366. Ab00052012
367. Dutoprol Component Hydrochlorothiazide
368. Eu-0100614
369. Ft-0650564
370. H1274
371. Prinzide Component Hydrochlorothiazide
372. Rasitrio Component Hydrochlorothiazide
373. Sw196569-3
374. Uniretic Component Hydrochlorothiazide
375. Accuretic Component Hydrochlorothiazide
376. Amturnide Component Hydrochlorothiazide
377. Apresazide Component Hydrochlorothiazide
378. Cam-ap-es Component Hydrochlorothiazide
379. H.r.-50 Component Hydrochlorothiazide
380. Hydrap-es Component Hydrochlorothiazide
381. Hydrochlorothiazde Component Of Toulomi
382. Hydrochlorothiazide 100 Microg/ml In Methanol
383. Hydrochlorothiazide Component Of Esimil
384. Hydrochlorothiazide Component Of Hyzaar
385. Hydropres Component Hydrochlorothiazide
386. Kervezide Component Hydrochlorothiazide
387. Kinzalkomb Component Hydrochlorothiazide
388. Moduretic Component Hydrochlorothiazide
389. Normozide Component Hydrochlorothiazide
390. Pritorplus Component Hydrochlorothiazide
391. Quinaretic Component Hydrochlorothiazide
392. Ser-a-gen Component Hydrochlorothiazide
393. Ser-ap-es Component Hydrochlorothiazide
394. Tolucombi Component Hydrochlorothiazide
395. Tribenzor Component Hydrochlorothiazide
396. Vaseretic Component Hydrochlorothiazide
397. Viskazide Component Hydrochlorothiazide
398. Zestoretic Component Hydrochlorothiazide
399. 6-chloro-3,2,4-benzothiadiazine 1,1-dioxide
400. A19550
401. Aldactazide Component Hydrochlorothiazide
402. Benicar Hct Component Hydrochlorothiazide
403. C07041
404. D00340
405. Diovan Hct Component Hydrochlorothiazide
406. Exforge Hct Component Hydrochlorothiazide
407. H10742
408. Hydra-zide Component Hydrochlorothiazide
409. Hydro-ride Component Hydrochlorothiazide
410. Hydrochlorothiazide Component Of Aldoril
411. Hydrochlorothiazide Component Of Avalide
412. Hydrochlorothiazide Component Of Dutoprol
413. Hydrochlorothiazide Component Of Dyazide
414. Hydrochlorothiazide Component Of Maxzide
415. Hydrochlorothiazide Component Of Prinzide
416. Hydrochlorothiazide Component Of Rasitrio
417. Hydrochlorothiazide Component Of Riprazo
418. Hydrochlorothiazide Component Of Unipres
419. Hydrochlorothiazide Component Of Uniretic
420. Ifirmacombi Component Hydrochlorothiazide
421. Sprimeo-hct Component Hydrochlorothiazide
422. Teveten Hct Component Hydrochlorothiazide
423. Ab00052012-15
424. Ab00052012_16
425. Ab00052012_17
426. Hydro-reserp Component Hydrochlorothiazide
427. Hydrochlorothiazide Component Of Amturnide
428. Hydrochlorothiazide Component Of H.r.-50
429. Hydrochlorothiazide Component Of Hydrap-es
430. Hydrochlorothiazide Component Of Hydropres
431. Hydrochlorothiazide Component Of Moduretic
432. Hydrochlorothiazide Component Of Normozide
433. Hydrochlorothiazide Component Of Tribenzor
434. Hydrochlorothiazide Component Of Vaseretic
435. Hydrochlorothiazide Component Of Viskazide
436. Lotensin Hct Component Hydrochlorothiazide
437. Micardis Hct Component Hydrochlorothiazide
438. Micardisplus Component Hydrochlorothiazide
439. Monopril Hct Component Hydrochlorothiazide
440. Tekturna Hct Component Hydrochlorothiazide
441. 051h765
442. Hydrochlorothiazide 100 Microg/ml In Acetonitrile
443. Hydrochlorothiazide Component Of Aldactazide
444. Hydrochlorothiazide Component Of Apresazide
445. Hydrochlorothiazide Component Of Benicar Hct
446. Hydrochlorothiazide Component Of Cam-ap-es
447. Hydrochlorothiazide Component Of Diovan Hct
448. Hydrochlorothiazide Component Of Exforge Hct
449. Hydrochlorothiazide Component Of Hydra-zide
450. Hydrochlorothiazide Component Of Hydro-ride
451. Hydrochlorothiazide Component Of Ifirmacombi
452. Hydrochlorothiazide Component Of Kinzalkomb
453. Hydrochlorothiazide Component Of Quinaretic
454. Hydrochlorothiazide Component Of Rasilez-hct
455. Hydrochlorothiazide Component Of Ser-a-gen
456. Hydrochlorothiazide Component Of Ser-ap-es
457. Hydrochlorothiazide Component Of Sprimeo-hct
458. Hydrochlorothiazide Component Of Teveten Hct
459. Hydrochlorothiazide Component Of Zestoretic
460. Lopressor Hct Component Hydrochlorothiazide
461. Oreticyl Forte Component Hydrochlorothiazide
462. Q423930
463. Hydrochlorothiazide Component Of Hydro-reserp
464. Hydrochlorothiazide Component Of Lotensin Hct
465. Hydrochlorothiazide Component Of Micardis Hct
466. Hydrochlorothiazide Component Of Micardisplus
467. Hydrochlorothiazide Component Of Monopril Hct
468. Hydrochlorothiazide Component Of Tekturna Hct
469. Hydrochlorothiazide, Meets Usp Testing Specifications
470. Q-201210
471. Serpasil-esidrix Component Hydrochlorothiazide
472. Sr-01000000119-2
473. Sr-01000000119-4
474. Sr-01000000119-6
475. Apresoline-esidrix Component Hydrochlorothiazide
476. Brd-k13078532-001-05-2
477. Hydrochlorothiazide Component Of Lopressor Hct
478. Hydrochlorothiazide Component Of Oreticyl Forte
479. Z56347248
480. Hydrochlorothiazide Component Of Serpasil-esidrix
481. 6-chloro-3,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide
482. 6-chloro-7-sulfamoyl-3,2,4-benzothiadiazine 1,1-dioxide
483. Hydrochlorothiazide Component Of Apresoline-esidrix
484. Hydrochlorothiazide, Drug Standard, 1.0 Mg/ml In Methanol
485. Hydroserpine Plus (r-h-h) Component Hydrochlorothiazide
486. 6-chloro-3,4-dihydro-1,1-dioxo-7-sulfamoyl-1,2,4-benzothiadiazine
487. Hydrochlorothiazide Component Of Hydroserpine Plus (r-h-h)
488. Hydrochlorothiazide, British Pharmacopoeia (bp) Reference Standard
489. Hydrochlorothiazide, European Pharmacopoeia (ep) Reference Standard
490. 3,4-dihydro-6-chloro-7-sulfamyl-1,2, 4-benzothiadiazine-1,1-dioxide
491. 6-chloro-3,4-dihydro-2h-1,2,4-benzothiadiazine-7-sulphonamide-1,1-dioxide
492. 6-chloro-3,4-dihydro-7-sulfamoyl-2h-1,2, 4-benzothiadiazine 1,1-dioxide
493. 6-chloro-7-sulfamoyl-3, 4-dihydro-2h-1,2,4-benzothiadiazine 1,1-dioxide
494. 6-chloro-7-sulfamoyl-3,4-dihydro-(2h)-1,2,4-benzothiadiazine 1,1-dioxide
495. 6-chloro-7-sulfamoyl-3,4-dihydrobenzo-1,2,4-thiadiazine-1,1-dioxide
496. 6-chloro-7-sulfamyl-3,4-dihydro-1,2,4-benzothiadiazine-1,1-dioxide
497. Hydrochlorothiazide Component Of Irbesartan/hydrochlorothiazide Teva
498. Hydrochlorothiazide Component Of Irbesartan/hydrochlorothiazide-bms
499. Hydrochlorothiazide, United States Pharmacopeia (usp) Reference Standard
500. Ibersartan/hydrochlorothiazide Zentiva Component Hydrochlorothiazide
501. Irbesartan/hydrochlorothiazide Teva Component Hydrochlorothiazide
502. Irbesartan/hydrochlorothiazide-bms Component Hydrochlorothiazide
503. 2h-1,2, 4-benzothiadiazine-7-sulfonamide, 6-chloro-3,4-dihydro-, 1, 1-dioxide
504. 2h-1,2,4-benzothiadiazine, 6-chloro-3,4-dihydro-7-sulfamoyl-2, 1,1-dioxide
505. 2h-1,2,4-benzothiadiazine-7-sulfonamide, 6-chloro-3,4-dihydro-,1,1-dioxide
506. 2h-1,4-benzothiadiazine-7-sulfonamide, 6-chloro-3,4-dihydro-, 1,1-dioxide
507. 6-chloro-1,1-dioxo-1,2,3,4-tetrahydro-1lambda,2,4-benzothiadiazine-7-sulfonamide
508. 6-chloro-1,1-dioxo-3,4-dihydro-2h-1$l^{6,2,4-benzothiadiazine-7-sulfonamide
509. 6-chloro-3,4-dihydro-2h-1,2,4-benzothiadiazine-7-sulfonamide 1, 1-dioxide
510. 7-(aminosulfonyl)-6-chloro-3,4-dihydro-(2h)-1,2,4-benzothiadiazine 1,1-dioxide
511. Hydralazine Hydrochloride-hydrochlorothiazide-reserpine Component Hydrochlorothiazide
512. Hydrochlorothiazide Component Of Ibersartan/hydrochlorothiazide Zentiva
513. Hydrochlorothiazide For Peak Identification, European Pharmacopoeia (ep) Reference Standard
514. Hydrochlorothiazide, Pharmaceutical Secondary Standard; Certified Reference Material
515. 125727-50-6
516. Hydrochlorothiazide Component Of Hydralazine Hydrochloride-hydrochlorothiazide-reserpine
517. Hydrochlorothiazide Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
| Molecular Weight | 297.7 g/mol |
|---|---|
| Molecular Formula | C7H8ClN3O4S2 |
| XLogP3 | -0.1 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 1 |
| Exact Mass | 296.9644758 g/mol |
| Monoisotopic Mass | 296.9644758 g/mol |
| Topological Polar Surface Area | 135 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 494 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 12 | |
|---|---|
| Drug Name | Dyazide |
| PubMed Health | Triamterene/Hydrochlorothiazide (By mouth) |
| Drug Classes | Diuretic, Potassium Sparing/Thiazide Combination |
| Active Ingredient | Hydrochlorothiazide; triamterene |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 37.5mg; 25mg |
| Market Status | Prescription |
| Company | Glaxosmithkline |
| 2 of 12 | |
|---|---|
| Drug Name | Hydrochlorothiazide |
| PubMed Health | Hydrochlorothiazide (By mouth) |
| Drug Classes | Cardiovascular Agent |
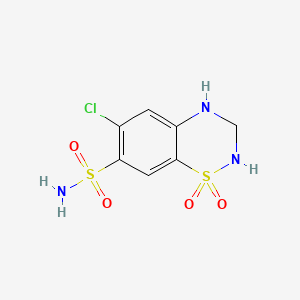
| Drug Label | Hydrochlorothiazide is a diuretic and antihypertensive. It is the 3,4-dihydro derivative of chlorothiazide. It is chemically designated as 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine -7-sulfonamide 1,1-dioxide and has the following structural form... |
| Active Ingredient | Hydrochlorothiazide |
| Dosage Form | Tablet; Capsule |
| Route | Oral |
| Strength | 25mg; 50mg; 12.5mg |
| Market Status | Prescription |
| Company | Vintage Pharms; Mylan Pharms; Excellium; Unichem; Jubilant Cadista; Apotex; Accord Hlthcare; Alembic Pharms; Sciegen Pharms; Sun Pharm Inds; Aurobindo Pharma; Hikma Pharms; Dava Pharms; Lannett Holdings; Ivax Sub Teva Pharms; Actavis Elizabeth; Ipca Labs; |
| 3 of 12 | |
|---|---|
| Drug Name | Lotensin hct |
| PubMed Health | Benazepril/Hydrochlorothiazide (By mouth) |
| Drug Classes | ACE Inhibitor/Thiazide Combination, Antihypertensive, Cardiovascular Agent |
| Active Ingredient | Benazepril hydrochloride; hydrochlorothiazide |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 25mg; 5mg; 6.25mg; 10mg; 12.5mg; 20mg |
| Market Status | Prescription |
| Company | Us Pharms Holdings I |
| 4 of 12 | |
|---|---|
| Drug Name | Microzide |
| PubMed Health | Hydrochlorothiazide (By mouth) |
| Drug Classes | Cardiovascular Agent |
| Drug Label | MICROZIDE (hydrochlorothiazide, USP)Capsules 12.5 mg is the 3,4-dihydro derivative of chlorothiazide. Its chemical name is 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide. Its empirical formula is C7H8ClN3O4S2; its mole... |
| Active Ingredient | Hydrochlorothiazide |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 12.5mg |
| Market Status | Prescription |
| Company | Watson Labs |
| 5 of 12 | |
|---|---|
| Drug Name | Oretic |
| PubMed Health | Lisinopril/Hydrochlorothiazide (By mouth) |
| Drug Classes | ACE Inhibitor/Thiazide Combination, Antihypertensive, Cardiovascular Agent |
| Active Ingredient | Hydrochlorothiazide |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 50mg |
| Market Status | Prescription |
| Company | Abbvie |
| 6 of 12 | |
|---|---|
| Drug Name | Prinzide |
| Active Ingredient | Hydrochlorothiazide; lisinopril |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 10mg; 12.5mg; 20mg |
| Market Status | Prescription |
| Company | Merck |
| 7 of 12 | |
|---|---|
| Drug Name | Dyazide |
| PubMed Health | Triamterene/Hydrochlorothiazide (By mouth) |
| Drug Classes | Diuretic, Potassium Sparing/Thiazide Combination |
| Active Ingredient | Hydrochlorothiazide; triamterene |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 37.5mg; 25mg |
| Market Status | Prescription |
| Company | Glaxosmithkline |
| 8 of 12 | |
|---|---|
| Drug Name | Hydrochlorothiazide |
| PubMed Health | Hydrochlorothiazide (By mouth) |
| Drug Classes | Cardiovascular Agent |
| Drug Label | Hydrochlorothiazide is a diuretic and antihypertensive. It is the 3,4-dihydro derivative of chlorothiazide. It is chemically designated as 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine -7-sulfonamide 1,1-dioxide and has the following structural form... |
| Active Ingredient | Hydrochlorothiazide |
| Dosage Form | Tablet; Capsule |
| Route | Oral |
| Strength | 25mg; 50mg; 12.5mg |
| Market Status | Prescription |
| Company | Vintage Pharms; Mylan Pharms; Excellium; Unichem; Jubilant Cadista; Apotex; Accord Hlthcare; Alembic Pharms; Sciegen Pharms; Sun Pharm Inds; Aurobindo Pharma; Hikma Pharms; Dava Pharms; Lannett Holdings; Ivax Sub Teva Pharms; Actavis Elizabeth; Ipca Labs; |
| 9 of 12 | |
|---|---|
| Drug Name | Lotensin hct |
| PubMed Health | Benazepril/Hydrochlorothiazide (By mouth) |
| Drug Classes | ACE Inhibitor/Thiazide Combination, Antihypertensive, Cardiovascular Agent |
| Active Ingredient | Benazepril hydrochloride; hydrochlorothiazide |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 25mg; 5mg; 6.25mg; 10mg; 12.5mg; 20mg |
| Market Status | Prescription |
| Company | Us Pharms Holdings I |
| 10 of 12 | |
|---|---|
| Drug Name | Microzide |
| PubMed Health | Hydrochlorothiazide (By mouth) |
| Drug Classes | Cardiovascular Agent |
| Drug Label | MICROZIDE (hydrochlorothiazide, USP)Capsules 12.5 mg is the 3,4-dihydro derivative of chlorothiazide. Its chemical name is 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide. Its empirical formula is C7H8ClN3O4S2; its mole... |
| Active Ingredient | Hydrochlorothiazide |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 12.5mg |
| Market Status | Prescription |
| Company | Watson Labs |
| 11 of 12 | |
|---|---|
| Drug Name | Oretic |
| PubMed Health | Lisinopril/Hydrochlorothiazide (By mouth) |
| Drug Classes | ACE Inhibitor/Thiazide Combination, Antihypertensive, Cardiovascular Agent |
| Active Ingredient | Hydrochlorothiazide |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 50mg |
| Market Status | Prescription |
| Company | Abbvie |
| 12 of 12 | |
|---|---|
| Drug Name | Prinzide |
| Active Ingredient | Hydrochlorothiazide; lisinopril |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 10mg; 12.5mg; 20mg |
| Market Status | Prescription |
| Company | Merck |
Antihypertensive Agents; Diuretics; Sodium Chloride Symporter Inhibitors
National Library of Medicine's Medical Subject Headings. Hydrochlorothiazide. Online file (MeSH, 2018). Available from, as of August 29, 2018: https://meshb.nlm.nih.gov/search
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Hydrochlorothiazide is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of August 29, 2018: https://clinicaltrials.gov/
Hydrochlorothiazide tablets, USP are indicated as adjunctive therapy in edema associated with congestive heart failure, hepatic cirrhosis, and corticosteroid and estrogen therapy. /Included in US product label/
NIH; DailyMed. Current Medication Information for Hydrochlorothiazide tablet (Updated: September 13, 2017). Available from, as of September 11, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=01f1f478-5493-439f-9b99-f4f82023781c
Hydrochlorothiazide tablets, USP have also been found useful in edema due to various forms of renal dysfunction such as nephrotic syndrome, acute glomerulonephritis, and chronic renal failure. /Included in US product label/
NIH; DailyMed. Current Medication Information for Hydrochlorothiazide tablet (Updated: September 13, 2017). Available from, as of September 11, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=01f1f478-5493-439f-9b99-f4f82023781c
For more Therapeutic Uses (Complete) data for Hydrochlorothiazide (7 total), please visit the HSDB record page.
Hydrochlorothiazide shares the pharmacologic actions, uses, and toxic potentials of the thiazides, and the usual precautions of thiazide administration should be observed.
American Society of Health-System Pharmacists; Drug Information 2018. Bethesda, MD. 2018, p. 2888
Some commercially available formulations of hydrochlorothiazide contain sulfites that may cause allergic-type reactions, including anaphylaxis and life-threatening or less severe asthmatic episodes, in certain susceptible individuals. The overall prevalence of sulfite sensitivity in the general population is unknown but probably low; such sensitivity appears to occur more frequently in asthmatic than in nonasthmatic individuals.
American Society of Health-System Pharmacists; Drug Information 2018. Bethesda, MD. 2018, p. 2888
The following adverse reactions have been reported and, within each category, are listed in the order of decreasing severity. ... Whenever adverse reactions are moderate or severe, thiazide dosage should be reduced or therapy withdrawn.
Table: Adverse Effects with Hydrochlorothiazide Tablet [Table#4434]
NIH; DailyMed. Current Medication Information for Hydrochlorothiazide tablet (Updated: September 13, 2017). Available from, as of September 11, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=01f1f478-5493-439f-9b99-f4f82023781c
The most common signs and symptoms /of overdose/ observed are those caused by electrolyte depletion (hypokalemia, hypochloremia, hyponatremia) and dehydration resulting from excessive diuresis. If digitalis has also been administered, hypokalemia may accentuate cardiac arrhythmias.
NIH; DailyMed. Current Medication Information for Hydrochlorothiazide tablet (Updated: September 13, 2017). Available from, as of September 11, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=01f1f478-5493-439f-9b99-f4f82023781c
For more Drug Warnings (Complete) data for Hydrochlorothiazide (28 total), please visit the HSDB record page.
Hydrochlorothiazide is indicated alone or in combination for the management of edema associated with congestive heart failure, hepatic cirrhosis, nephrotic syndrome, acute glomerulonephritis, chronic renal failure, and corticosteroid and estrogen therapy. Hydrochlorothiazide is also indicated alone or in combination for the management of hypertension.
Hydrochlorothiazide prevents the reabsorption of sodium and water from the distal convoluted tubule, allowing for the increased elimination of water in the urine. Hydrochlorothiazide has a wide therapeutic window as dosing is individualized and can range from 25-100mg. Hydrochlorothiazide should be used with caution in patients with reduced kidney or liver function.
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
Diuretics
Agents that promote the excretion of urine through their effects on kidney function. (See all compounds classified as Diuretics.)
Sodium Chloride Symporter Inhibitors
Agents that inhibit SODIUM CHLORIDE SYMPORTERS. They act as DIURETICS. Excess use is associated with HYPOKALEMIA. (See all compounds classified as Sodium Chloride Symporter Inhibitors.)
C09DX03
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
C03AA03
S66 | EAWAGTPS | Parent-Transformation Product Pairs from Eawag | DOI:10.5281/zenodo.3754448
C03AA03
S66 | EAWAGTPS | Parent-Transformation Product Pairs from Eawag | DOI:10.5281/zenodo.3754448
C03AA03
S66 | EAWAGTPS | Parent-Transformation Product Pairs from Eawag | DOI:10.5281/zenodo.3754448
C03AA03
S66 | EAWAGTPS | Parent-Transformation Product Pairs from Eawag | DOI:10.5281/zenodo.3754448
C - Cardiovascular system
C03 - Diuretics
C03A - Low-ceiling diuretics, thiazides
C03AA - Thiazides, plain
C03AA03 - Hydrochlorothiazide
Absorption
An oral dose of hydrochlorothiazide is 65-75% bioavailable, with a Tmax of 1-5 hours, and a Cmax of 70-490ng/mL following doses of 12.5-100mg. When taken with a meal, bioavailability is 10% lower, Cmax is 20% lower, and Tmax increases from 1.6 to 2.9 hours.
Route of Elimination
Hydrochlorothiazide is eliminated in the urine as unchanged hydrochlorothiazide.
Volume of Distribution
The volume of distribution varies widely from one study to another with values of 0.83-4.19L/kg.
Clearance
The renal clearance of hydrochlorothiazide in patients with normal renal function is 285mL/min. Patients with a creatinine clearance of 31-80mL/min have an average hydroxychlorothiazide renal clearance of 75mL/min, and patients with a creatinine clearance of 30mL/min have an average hydroxychlorothiazide renal clearance of 17mL/min.
Hydrochlorothiazide is well absorbed from the GI tract, with an oral bioavailability of approximately 65-75%. Although the rate and extent of absorption have been reported to vary depending on the formulation administered, no studies have been performed to determine the clinical importance (if any) of variations in absorption in patients receiving chronic hydrochlorothiazide therapy. Following oral administration of hydrochlorothiazide at doses of 12.5-100 mg, peak plasma concentrations of 70-490 ng/mL are observed within 1-5 hours of dosing. Food decreases the rate and extent of absorption of hydrochlorothiazide capsules (Microzide). Bioavailability and peak plasma concentrations of the drug were decreased by about 10 and 20%, respectively, when hydrochlorothiazide capsules (Microzide) were administered with food. Times to peak plasma concentration for such capsules were delayed by 1.3 hours (from 1.6 to 2.9 hours). Absorption of hydrochlorothiazide is reduced in patients with heart failure. Approximately 40-68% of the drug is bound to plasma proteins. Hydrochlorothiazide exhibits linear pharmacokinetics. Based on determination of plasma drug concentrations over a period of at least 24 hours, the plasma half-life of hydrochlorothiazide reportedly ranges from 5.6-15 hours. Hydrochlorothiazide apparently is not metabolized and is excreted unchanged in urine. At least 61% of the drug is reportedly eliminated from the body within 24 hours. Increased hydrochlorothiazide plasma concentrations and a prolonged elimination half-life have been reported in patients with renal impairment. The effect of hemodialysis on the elimination of the drug has not been determined.
American Society of Health-System Pharmacists; Drug Information 2018. Bethesda, MD. 2018, p. 2888
Thiazides cross the placental barrier and appear in cord blood. /Thiazides/
NIH; DailyMed. Current Medication Information for Hydrochlorothiazide tablet (Updated: September 13, 2017). Available from, as of September 11, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=01f1f478-5493-439f-9b99-f4f82023781c
/MILK/ Thiazides are excreted in breast milk. /Thiazides/
NIH; DailyMed. Current Medication Information for Hydrochlorothiazide tablet (Updated: September 13, 2017). Available from, as of September 11, 2018: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=01f1f478-5493-439f-9b99-f4f82023781c
(14)C-hydrochlorothiazide (hct) was administered orally (n=4) and iv (n=2) to healthy subjects. The gastrointestinal absorption ranged between 60% and 80%, most of it took place in the duodenum and the upper jejunum. The radioactivity was eliminated mainly in the urine, while no sigificant biliary excretion was observed. Chromatographic analysis of the urinary radioactivity demonstrated that greater than 95% of the absorbed or injected (14)C-hct was excreted unchanged. The radioactivity in plasma during the first 10 hr after oral administration declined with a fast phase but the levels of label thereafter suggested a slow phase. The existence of such a phase was verified in 1 subject given 75 mg hct orally. His plasma levels of hct (determined with gas-liquid chromatography) declined according to a 2-compartment model, the half-lives of the alpha-and beta-phases being 1.7 and 13.1 hr, respectively. Hct accumulated in the blood cells and the ratio between the radioactivity in cells and that in plasma averaged 3.5. The fate of a single dose of (14)C-hct in 2 hypertensive patients treated with the drug chronically was similar to that in the healthy subjects. A third patient, who had slightly elevated serum creatinine, eliminated hct more slowly than the others. Like the healthy subjects, the patients eliminated hct to greater than 95% in unchanged form.
PMID:1277708 Beermann B et al; Clin Pharmacol Ther 19 (5 Pt 1): 531-7 (1976)
For more Absorption, Distribution and Excretion (Complete) data for Hydrochlorothiazide (6 total), please visit the HSDB record page.
Hydrochlorothiazide is not metabolized.
The plasma half life of hydrochlorothiazide is 5.6-14.8h.
Based on determination of plasma drug concentrations over a period of at least 24 hours, the plasma half-life of hydrochlorothiazide reportedly ranges from 5.6-15 hours.
American Society of Health-System Pharmacists; Drug Information 2018. Bethesda, MD. 2018, p. 2888
The bioavailability of hydrochlorothiazide from 50-mg oral tablet doses was examined in healthy male volunteers under fasting and nonfasting conditions. ... The pharmacokinetics of hydrochlorothiazide in plasma could be described in terms of a triexponential function, and the mean half-life determined from the 3 exponents were 1.0, 2.2, and 9.0 hr.
PMID:7062255 Barbhaiya RH et al; J Pharm Sci 71 (2): 245-8 (1982)
The radioactivity in /human/ plasma during the first 10 hr after oral administration /of hydrochlorothiazide (hct)/ declined with a fast phase but the levels of label thereafter suggested a slow phase. The existence of such a phase was verified in 1 subject given 75 mg hct orally. His plasma levels of hct (determined with gas-liquid chromatography) declined according to a 2-compartment model, the half-lives of the alpha-and beta-phases being 1.7 and 13.1 hr, respectively.
PMID:1277708 Beermann B et al; Clin Pharmacol Ther 19 (5 Pt 1): 531-7 (1976)
Hydrochlorothiazide is transported from the circulation into epithelial cells of the distal convoluted tubule by the organic anion transporters OAT1, OAT3, and OAT4. From these cells, hydrochlorothiazide is transported to the lumen of the tubule by multidrug resistance associated protein 4 (MRP4). Normally, sodium is reabsorbed into epithelial cells of the distal convoluted tubule and pumped into the basolateral interstitium by a sodium-potassium ATPase, creating a concentration gradient between the epithelial cell and the distal convoluted tubule that promotes the reabsorption of water. Hydrochlorothiazide acts on the proximal region of the distal convoluted tubule, inhibiting reabsorption by the sodium-chloride symporter, also known as Solute Carrier Family 12 Member 3 (SLC12A3). Inhibition of SLC12A3 reduces the magnitude of the concentration gradient between the epithelial cell and distal convoluted tubule, reducing the reabsorption of water.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
24
PharmaCompass offers a list of Hydrochlorothiazide API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Hydrochlorothiazide manufacturer or Hydrochlorothiazide supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Hydrochlorothiazide manufacturer or Hydrochlorothiazide supplier.
PharmaCompass also assists you with knowing the Hydrochlorothiazide API Price utilized in the formulation of products. Hydrochlorothiazide API Price is not always fixed or binding as the Hydrochlorothiazide Price is obtained through a variety of data sources. The Hydrochlorothiazide Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Hydrochlorothiazide manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Hydrochlorothiazide, including repackagers and relabelers. The FDA regulates Hydrochlorothiazide manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Hydrochlorothiazide API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Hydrochlorothiazide manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Hydrochlorothiazide supplier is an individual or a company that provides Hydrochlorothiazide active pharmaceutical ingredient (API) or Hydrochlorothiazide finished formulations upon request. The Hydrochlorothiazide suppliers may include Hydrochlorothiazide API manufacturers, exporters, distributors and traders.
click here to find a list of Hydrochlorothiazide suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Hydrochlorothiazide DMF (Drug Master File) is a document detailing the whole manufacturing process of Hydrochlorothiazide active pharmaceutical ingredient (API) in detail. Different forms of Hydrochlorothiazide DMFs exist exist since differing nations have different regulations, such as Hydrochlorothiazide USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Hydrochlorothiazide DMF submitted to regulatory agencies in the US is known as a USDMF. Hydrochlorothiazide USDMF includes data on Hydrochlorothiazide's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Hydrochlorothiazide USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Hydrochlorothiazide suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Hydrochlorothiazide Drug Master File in Japan (Hydrochlorothiazide JDMF) empowers Hydrochlorothiazide API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Hydrochlorothiazide JDMF during the approval evaluation for pharmaceutical products. At the time of Hydrochlorothiazide JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Hydrochlorothiazide suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Hydrochlorothiazide Drug Master File in Korea (Hydrochlorothiazide KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Hydrochlorothiazide. The MFDS reviews the Hydrochlorothiazide KDMF as part of the drug registration process and uses the information provided in the Hydrochlorothiazide KDMF to evaluate the safety and efficacy of the drug.
After submitting a Hydrochlorothiazide KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Hydrochlorothiazide API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Hydrochlorothiazide suppliers with KDMF on PharmaCompass.
A Hydrochlorothiazide CEP of the European Pharmacopoeia monograph is often referred to as a Hydrochlorothiazide Certificate of Suitability (COS). The purpose of a Hydrochlorothiazide CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Hydrochlorothiazide EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Hydrochlorothiazide to their clients by showing that a Hydrochlorothiazide CEP has been issued for it. The manufacturer submits a Hydrochlorothiazide CEP (COS) as part of the market authorization procedure, and it takes on the role of a Hydrochlorothiazide CEP holder for the record. Additionally, the data presented in the Hydrochlorothiazide CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Hydrochlorothiazide DMF.
A Hydrochlorothiazide CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Hydrochlorothiazide CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Hydrochlorothiazide suppliers with CEP (COS) on PharmaCompass.
A Hydrochlorothiazide written confirmation (Hydrochlorothiazide WC) is an official document issued by a regulatory agency to a Hydrochlorothiazide manufacturer, verifying that the manufacturing facility of a Hydrochlorothiazide active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Hydrochlorothiazide APIs or Hydrochlorothiazide finished pharmaceutical products to another nation, regulatory agencies frequently require a Hydrochlorothiazide WC (written confirmation) as part of the regulatory process.
click here to find a list of Hydrochlorothiazide suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Hydrochlorothiazide as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Hydrochlorothiazide API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Hydrochlorothiazide as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Hydrochlorothiazide and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Hydrochlorothiazide NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Hydrochlorothiazide suppliers with NDC on PharmaCompass.
Hydrochlorothiazide Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Hydrochlorothiazide GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Hydrochlorothiazide GMP manufacturer or Hydrochlorothiazide GMP API supplier for your needs.
A Hydrochlorothiazide CoA (Certificate of Analysis) is a formal document that attests to Hydrochlorothiazide's compliance with Hydrochlorothiazide specifications and serves as a tool for batch-level quality control.
Hydrochlorothiazide CoA mostly includes findings from lab analyses of a specific batch. For each Hydrochlorothiazide CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Hydrochlorothiazide may be tested according to a variety of international standards, such as European Pharmacopoeia (Hydrochlorothiazide EP), Hydrochlorothiazide JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Hydrochlorothiazide USP).
