
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
VMF
0
Europe

0
Australia

0
South Africa

0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
Finished Drug Prices
NA
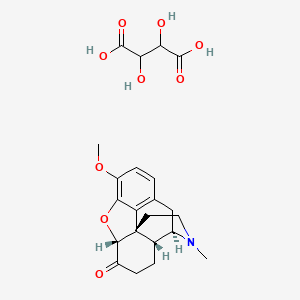

1. Codinovo
2. Dicodid
3. Dihydrocodeinone
4. Hycodan
5. Hycon
6. Hydrocodeinonebitartrate
7. Hydrocodon
8. Hydrocodone
9. Hydrocodone Tartrate (1:1), Hydrate (2:5)
10. Hydrocon
11. Robidone
1. (4r,4ar,7ar,12bs)-9-methoxy-3-methyl-1,2,4,4a,5,6,7a,13-octahydro-4,12-methanobenzofuro[3,2-e]isoquinolin-7-one;2,3-dihydroxybutanedioic Acid
2. .codon
3. Schembl154972
4. Chembl2062267
1. Hydrocone
2. Multacodin
3. Hydrocodone
| Molecular Weight | 449.4 g/mol |
|---|---|
| Molecular Formula | C22H27NO9 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 4 |
| Exact Mass | 449.16858144 g/mol |
| Monoisotopic Mass | 449.16858144 g/mol |
| Topological Polar Surface Area | 154 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 642 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 2 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Analgesics, Opioid
Compounds with activity like OPIATE ALKALOIDS, acting at OPIOID RECEPTORS. Properties include induction of ANALGESIA or NARCOSIS. (See all compounds classified as Analgesics, Opioid.)
Antitussive Agents
Agents that suppress cough. They act centrally on the medullary cough center. EXPECTORANTS, also used in the treatment of cough, act locally. (See all compounds classified as Antitussive Agents.)
Narcotics
Agents that induce NARCOSIS. Narcotics include agents that cause somnolence or induced sleep (STUPOR); natural or synthetic derivatives of OPIUM or MORPHINE or any substance that has such effects. They are potent inducers of ANALGESIA and OPIOID-RELATED DISORDERS. (See all compounds classified as Narcotics.)
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?