
Synopsis
Synopsis
0
KDMF
0
VMF
DRUG PRODUCT COMPOSITIONS
Annual Reports
NA
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
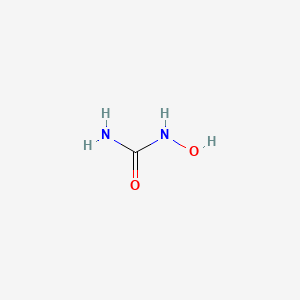

1. Hydrea
2. Hydroxycarbamid
3. Oncocarbide
1. Hydroxycarbamide
2. 127-07-1
3. N-hydroxyurea
4. Hydrea
5. 1-hydroxyurea
6. Oxyurea
7. Carbamoyl Oxime
8. Biosupressin
9. Hydroxycarbamine
10. Urea, Hydroxy-
11. Onco-carbide
12. Carbamohydroxamic Acid
13. Carbamohydroximic Acid
14. Carbamyl Hydroxamate
15. Droxia
16. Hydura
17. Litalir
18. Hydurea
19. N-carbamoylhydroxylamine
20. Hidrix
21. Siklos
22. Hydroxicarbamidum
23. Hydroxylurea
24. Hydreia
25. Litaler
26. Idrossicarbamide [dcit]
27. Hidroxicarbamida
28. Hydroxyharnstoff
29. Sq 1089
30. Hydroxycarbamidum
31. Carbamohydroxyamic Acid
32. N-hydroxymocovina
33. Hydroxylamine, N-carbamoyl-
34. Hydroxyharnstoff [german]
35. N-hydroxymocovina [czech]
36. Hydroxy Urea
37. Nci-c04831
38. Hydroxylamine, N-(aminocarbonyl)-
39. Sk 22591
40. Hydroxycarbamidum [inn-latin]
41. Hidroxicarbamida [inn-spanish]
42. Urea, N-hydroxy-
43. Hu
44. Ccris 958
45. Hydroxy-urea
46. Nsc 32065
47. Nsc32065
48. Sq-1089
49. Ai3-51139
50. Hydroxyurea (cytodrox)
51. Hydroxycarbamide [inn]
52. Nsc-32065
53. Chembl467
54. X6q56qn5qc
55. Chebi:44423
56. Ncgc00015520-03
57. Hydroxycarbamid
58. Oncocarbide
59. Idrossicarbamide
60. Dsstox_cid_5438
61. Dsstox_rid_77787
62. Dsstox_gsid_25438
63. 8029-68-3
64. Nhy
65. Hydroxyurea (d4)
66. N-hydroxy Urea
67. Mylocel
68. Carbamide Oxide
69. Xromi
70. Cas-127-07-1
71. Smr000059149
72. Hydroxyurea (usp)
73. Droxia (tm)
74. Droxia (tn)
75. Hydrea (tm)
76. Hydroxyaminomethanamide
77. Hsdb 6887
78. Sr-01000075919
79. Drg-0253
80. Einecs 204-821-7
81. Hydrea (tn)
82. Mfcd00007943
83. Hydroxyurea [usan:usp]
84. Unii-x6q56qn5qc
85. Brn 1741548
86. Hydroxycarbamide (jan/inn)
87. Hydroxyl Urea
88. N-hydroxy-urea
89. S-phase/g-1 Interface Inhibitor
90. Aminohydroxamic Acid
91. Carbamic Acid Oxime
92. Carbomohydroxamic Acid
93. Spectrum_000909
94. Wln: Zvmq
95. Hydrea (bristol Meyers)
96. Hydroxyurea [mi]
97. Spectrum2_000064
98. Spectrum3_000462
99. Spectrum4_000012
100. Spectrum5_000836
101. Lopac-h-8627
102. Hydroxyurea [hsdb]
103. Hydroxyurea [iarc]
104. Hydroxyurea [usan]
105. Molmap_000029
106. H 8627
107. Hydroxyurea [vandf]
108. Ncimech_000139
109. Hydroxyurea, 98%, Powder
110. Lopac0_000596
111. Bspbio_002164
112. Hydroxyurea [usp-rs]
113. Kbiogr_000383
114. Kbioss_001389
115. 4-03-00-00170 (beilstein Handbook Reference)
116. Hydroxycarbamide (hydroxyurea)
117. Mls001332381
118. Mls001332382
119. Mls002153389
120. Divk1c_000556
121. Hydroxycarbamide [jan]
122. N-(aminocarbonyl)hydroxylamine
123. Spectrum1500344
124. Spbio_000247
125. Gtpl6822
126. Dtxsid6025438
127. Hydroxycarbamide [mart.]
128. Tetratogen: Inhibits Ribonucleoside Diphosphate Reductase
129. Hms501l18
130. Kbio1_000556
131. Kbio2_001389
132. Kbio2_003957
133. Kbio2_006525
134. Kbio3_001384
135. Hydroxycarbamide [who-dd]
136. Hydroxyurea [orange Book]
137. Ninds_000556
138. Bio1_000451
139. Bio1_000940
140. Bio1_001429
141. Hms1920f09
142. Hms2091l17
143. Hms2234i03
144. Hms3261h14
145. Hms3373g18
146. Hms3655k20
147. Hms3869c03
148. Nci C04831
149. Pharmakon1600-01500344
150. Hydroxycarbamide [ema Epar]
151. Hydroxyurea [usp Monograph]
152. Act02611
153. Albb-028465
154. Amy40858
155. Hy-b0313
156. Str02555
157. Zinc8034120
158. Tox21_110168
159. Tox21_300319
160. Tox21_500596
161. Bbl009928
162. Bdbm50017811
163. Ccg-35236
164. Nsc757072
165. S1896
166. Stl145898
167. Akos005716276
168. Akos006222547
169. Tox21_110168_1
170. Zinc100019199
171. Db01005
172. Hydroxycarbamide [ep Monograph]
173. Lp00596
174. Nsc-757072
175. Sdccgsbi-0050578.p006
176. Idi1_000556
177. Ncgc00015520-01
178. Ncgc00015520-02
179. Ncgc00015520-04
180. Ncgc00015520-05
181. Ncgc00015520-06
182. Ncgc00015520-07
183. Ncgc00015520-08
184. Ncgc00015520-09
185. Ncgc00015520-10
186. Ncgc00015520-11
187. Ncgc00015520-20
188. Ncgc00093974-01
189. Ncgc00093974-02
190. Ncgc00093974-03
191. Ncgc00093974-04
192. Ncgc00093974-05
193. Ncgc00254007-01
194. Ncgc00261281-01
195. Ac-22674
196. Nci60_002773
197. Sbi-0050578.p004
198. Db-041849
199. Eu-0100596
200. Ft-0627160
201. Ft-0627175
202. Ft-0670210
203. H0310
204. Sw218071-2
205. C07044
206. D00341
207. Hydroxyurea, Vetec(tm) Reagent Grade, >=98%
208. Ab00052018-09
209. Ab00052018-10
210. Ab00052018_11
211. Ab00052018_12
212. 127h071
213. A805636
214. Q212272
215. J-504798
216. Sr-01000075919-1
217. Sr-01000075919-3
218. Sr-01000075919-8
219. E0723dba-5af3-49d1-b5f6-59420ab87ac9
220. F8880-0905
221. Z1522566612
222. Hydroxycarbamide, European Pharmacopoeia (ep) Reference Standard
223. Hydroxyurea, United States Pharmacopeia (usp) Reference Standard
| Molecular Weight | 76.055 g/mol |
|---|---|
| Molecular Formula | CH4N2O2 |
| XLogP3 | -1.8 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 76.027277375 g/mol |
| Monoisotopic Mass | 76.027277375 g/mol |
| Topological Polar Surface Area | 75.4 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 42.9 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Droxia |
| PubMed Health | Hydroxyurea (By mouth) |
| Drug Classes | Antineoplastic Agent |
| Drug Label | DROXIA (hydroxyurea capsules, USP) is available for oral use as capsules providing 200 mg, 300 mg, and 400 mg hydroxyurea. Inactive ingredients: citric acid, gelatin, lactose, magnesium stearate, sodium phosphate, titanium dioxide, and capsule colo... |
| Active Ingredient | Hydroxyurea |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 300mg; 200mg; 400mg |
| Market Status | Prescription |
| Company | Bristol Myers Squibb |
| 2 of 6 | |
|---|---|
| Drug Name | Hydrea |
| PubMed Health | Hydroxyurea (By mouth) |
| Drug Classes | Antineoplastic Agent |
| Drug Label | HYDREA (hydroxyurea capsules, USP) is an antineoplastic agent available for oral use as capsules providing 500 mg hydroxyurea. Inactive ingredients: citric acid, colorants (D&C Yellow No. 10, FD&C Blue No. 1, FD&C Red No. 40, and D&C Red N |
| Active Ingredient | Hydroxyurea |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 500mg |
| Market Status | Prescription |
| Company | Bristol Myers Squibb |
| 3 of 6 | |
|---|---|
| Drug Name | Hydroxyurea |
| PubMed Health | Hydroxyurea (By mouth) |
| Drug Classes | Antineoplastic Agent |
| Drug Label | DROXIA (hydroxyurea capsules, USP) is available for oral use as capsules providing 200 mg, 300 mg, and 400 mg hydroxyurea. Inactive ingredients: citric acid, gelatin, lactose, magnesium stearate, sodium phosphate, titanium dioxide, and capsule colo... |
| Active Ingredient | Hydroxyurea |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 500mg |
| Market Status | Prescription |
| Company | Par Pharm; Barr |
| 4 of 6 | |
|---|---|
| Drug Name | Droxia |
| PubMed Health | Hydroxyurea (By mouth) |
| Drug Classes | Antineoplastic Agent |
| Drug Label | DROXIA (hydroxyurea capsules, USP) is available for oral use as capsules providing 200 mg, 300 mg, and 400 mg hydroxyurea. Inactive ingredients: citric acid, gelatin, lactose, magnesium stearate, sodium phosphate, titanium dioxide, and capsule colo... |
| Active Ingredient | Hydroxyurea |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 300mg; 200mg; 400mg |
| Market Status | Prescription |
| Company | Bristol Myers Squibb |
| 5 of 6 | |
|---|---|
| Drug Name | Hydrea |
| PubMed Health | Hydroxyurea (By mouth) |
| Drug Classes | Antineoplastic Agent |
| Drug Label | HYDREA (hydroxyurea capsules, USP) is an antineoplastic agent available for oral use as capsules providing 500 mg hydroxyurea. Inactive ingredients: citric acid, colorants (D&C Yellow No. 10, FD&C Blue No. 1, FD&C Red No. 40, and D&C Red N |
| Active Ingredient | Hydroxyurea |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 500mg |
| Market Status | Prescription |
| Company | Bristol Myers Squibb |
| 6 of 6 | |
|---|---|
| Drug Name | Hydroxyurea |
| PubMed Health | Hydroxyurea (By mouth) |
| Drug Classes | Antineoplastic Agent |
| Drug Label | DROXIA (hydroxyurea capsules, USP) is available for oral use as capsules providing 200 mg, 300 mg, and 400 mg hydroxyurea. Inactive ingredients: citric acid, gelatin, lactose, magnesium stearate, sodium phosphate, titanium dioxide, and capsule colo... |
| Active Ingredient | Hydroxyurea |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 500mg |
| Market Status | Prescription |
| Company | Par Pharm; Barr |
Antineoplastic Agents; Antisickling Agents; Enzyme Inhibitors; Nucleic Acid Synthesis Inhibitors
National Library of Medicine's Medical Subject Headings. Hydroxyurea. Online file (MeSH, 2016). Available from, as of January 19, 2016: https://www.nlm.nih.gov/mesh/2016/mesh_browser/MBrowser.html
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Hydroxyurea is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of March 17, 2016: https://clinicaltrials.gov/ct2/results?term=hydroxyurea&Search=Search
Hydroxyurea Capsules USP are indicated for the treatment of: Resistant chronic myeloid leukemia. Locally advanced squamous cell carcinomas of the head and neck (excluding the lip) in combination with chemoradiation. /Included in US product label/
NIH; DailyMed. Current Medication Information for Hydroxyurea Capsule (Updated: January 2016). Available from, as of January 21, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b9514ae5-79ae-4cc2-9d7f-c8f7806d1694
Hydroxyurea has been used in the treatment of psoriasis and is reportedly beneficial in the treatment of hypereosinophilic syndrome that does not respond to corticosteroid therapy. /NOT included in US product label/
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 1061
For more Therapeutic Uses (Complete) data for HYDROXYUREA (12 total), please visit the HSDB record page.
Hydroxyurea is a highly toxic drug with a low therapeutic index, and a therapeutic response is not likely to occur without some evidence of toxicity. Hydroxyurea therapy may be complicated by severe, sometimes life-threatening or fatal, adverse effects. The drug must be used only under constant supervision by clinicians experienced in therapy with cytotoxic agents or the use of this agent for sickle cell anemia.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 1063
Hydroxyurea should be admin with caution to patients who have recently received other cytotoxic drugs or irradiation therapy, since bone marrow depression is likely in these patients. In addition, an exacerbation of post-irradiation erythema may occur.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 1063
Hepatotoxicity, in some cases resulting in fatal hepatic failure, has been reported in patients with HIV infection receiving hydroxyurea in combination with antiretroviral agents. Fatal hepatotoxicity occurred most frequently in patients receiving combination therapy with hydroxyurea, didanosine, and stavudine. Elevation of serum concentrations of hepatic enzymes has been reported in patients receiving hydroxyurea.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 1063
Cutaneous vasculitic toxicities, including vasculitic ulcerations and gangrene, have occurred in patients receiving hydroxyurea for myeloproliferative disorders, particularly in patients who have received or who are receiving interferon therapy.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 1063
For more Drug Warnings (Complete) data for HYDROXYUREA (37 total), please visit the HSDB record page.
For management of melanoma, resistant chronic myelocytic leukemia, and recurrent, metastatic, or inoperable carcinoma of the ovary and Sickle-cell anemia.
Siklos is indicated for the prevention of recurrent painful vaso-occlusive crises including acute chest syndrome in paediatric and adult patients suffering from symptomatic sickle-cell syndrome.
Prevention of vaso-occlusive complications of sickle cell disease in patients over 2 years of age
Hydroxyurea has dose-dependent synergistic activity with cisplatin in vitro. In vivo Hydroxyurea showed activity in combination with cisplatin against the LX-1 and CALU-6 human lung xenografts, but minimal activity was seen with the NCI-H460 or NCI-H520 xenografts. Hydroxyurea was synergistic with cisplatin in the Lewis lung murine xenograft. Sequential exposure to Hydroxyurea 4 hours before cisplatin produced the greatest interaction.
Antisickling Agents
Agents used to prevent or reverse the pathological events leading to sickling of erythrocytes in sickle cell conditions. (See all compounds classified as Antisickling Agents.)
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)
Nucleic Acid Synthesis Inhibitors
Compounds that inhibit cell production of DNA or RNA. (See all compounds classified as Nucleic Acid Synthesis Inhibitors.)
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
L01XX05
L01XX05
L01XX05
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01X - Other antineoplastic agents
L01XX - Other antineoplastic agents
L01XX05 - Hydroxycarbamide
Absorption
Well absorbed from the gastrointestinal tract.
Route of Elimination
Renal excretion is a pathway of elimination.
Hydroxyurea is readily absorbed from the GI tract. Peak serum concentrations are attained within 1-4 hours following oral administration. Blood concentrations decline rapidly and there is no cumulative effect with repeated administration. For this reason, higher blood concentrations are attained if the regular dosage is given in a large, single oral dose than if it is administered in divided doses. Disproportionate increases in peak plasma concentrations and areas under the concentration-time curve (AUCs) result when drug dosage is increased. The effect of food on the absorption of hydroxyurea has not been determined.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 1065
Hydroxyurea distributes rapidly throughout the body and concentrates in leukocytes and erythrocytes. The estimated volume of distribution of the drug approximates total body water. Hydroxyurea crosses the blood-brain barrier; peak hydroxyurea CSF concentrations are attained within 3 hours following oral administration. The drug distributes into ascites fluid, resulting in drug concentrations in ascites fluid of 2-7.5 times less than plasma drug concentrations.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 1065
Studies using(14)C-labeled hydroxyurea indicate that about one-half an orally administered dose is degraded in the liver and is excreted as respiratory carbon dioxide and in urine as urea. The remaining portion of the drug is excreted intact in urine.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 1065
About 30-60% of an orally administered dose of hydroxyurea is excreted unchanged by the kidneys, although about 35% is generally excreted.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V76 361 (2000)
For more Absorption, Distribution and Excretion (Complete) data for HYDROXYUREA (7 total), please visit the HSDB record page.
Hepatic.
Studies indicate that up to 50% of an orally administered dose of hydroxyurea is metabolized in the liver; however, the precise metabolic pathways have not been determined. A minor metabolic pathway may involve degradation of the drug by urease, an enzyme produced by intestinal bacteria. Acetohydroxamic acid, possibly resulting from the breakdown of hydroxyurea by urease, was detected in the serum of 3 patients with leukemia treated with hydroxyurea.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 1065
3-4 hours
The half-time of hydroxyurea is short, with an initial half-time of 0.63 hr after intravenous administration and 1.78 hr after oral administration and a terminal half-time of 3.32 hr after oral administration and 3.39 hr after intravenous administration /to humans/.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V76 360 (2000)
The half-time of hydroxyurea in rats given 137 mg/kg bw per day intraperitoneally on days 9-12 of gestation was 15 min in the dams and 85 min in the embryos. In rhesus monkeys given 100 mg/kg bw per day intravenously on days 23-32 of gestation, the half-time was 120 min after the last injection in the mothers and 265 min in their fetuses.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V76 362 (2000)
Hydroxyurea is converted to a free radical nitroxide (NO) in vivo, and transported by diffusion into cells where it quenches the tyrosyl free radical at the active site of the M2 protein subunit of ribonucleotide reductase, inactivating the enzyme. The entire replicase complex, including ribonucleotide reductase, is inactivated and DNA synthesis is selectively inhibited, producing cell death in S phase and synchronization of the fraction of cells that survive. Repair of DNA damaged by chemicals or irradiation is also inhibited by hydroxyurea, offering potential synergy between hydroxyurea and radiation or alkylating agents. Hydroxyurea also increases the level of fetal hemoglobin, leading to a reduction in the incidence of vasoocclusive crises in sickle cell anemia. Levels of fetal hemoglobin increase in response to activation of soluble guanylyl cyclase (sGC) by hydroxyurea-derived NO.
The exact mechanism of antineoplastic activity of hydroxyurea has not been fully determined. Some studies indicate that hydroxyurea interferes with the synthesis of DNA without interfering with the synthesis of RNA or protein. Although hydroxyurea may have multiple sites of action, it appears likely that the drug inhibits the incorporation of thymidine into DNA; in addition, it may directly damage DNA. Hydroxyurea can destroy the tyrosyl free radical that is formed as the catalytic center of ribonucleoside diphosphate reductase, the enzyme that catalyzes the reductive conversion of ribonucleotides to deoxyribonucleotides; this conversion is a critical and probably rate-limiting step in the synthesis of DNA. The drug is an S-phase inhibitor and may cause cells to arrest at the G1-S border, decrease the rate of cell progression into the S phase, and/or cause cells to accumulate in the S phase as a result of inhibiting DNA synthesis. Animal studies indicate that the cytotoxic effects of hydroxyurea are limited to those tissues with high rates of cellular proliferation and the effects are evident only in those cells that are actively synthesizing DNA.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 1064
Hydroxyurea, a drug widely used in therapy of several human diseases, inhibits deoxynucleotide synthesis and, consequently, DNA synthesis by blocking the cellular enzyme ribonucleotide reductase. Hydroxyurea inhibits human immunodeficiency virus type 1 (HIV-1) DNA synthesis in activated peripheral blood lymphocytes by decreasing the amount of intracellular deoxynucleotides, thus suggesting that this drug has an antiviral effect. Hydroxyurea has now been shown to block HIV-1 replication in acutely infected primary human lymphocytes (quiescent and activated) and macrophages, as well as in blood cells infected in vivo obtained from individuals with acquired immunodeficiency syndrome (AIDS). The antiviral effect was achieved at nontoxic doses of hydroxyurea, lower than those currently used in human therapy. Combination of hydroxyurea with the nucleoside analog didanosine (2'3'-dideoxyinosine, or ddi) generated a synergistic inhibitory effect without increasing toxicity. In some instances, inhibition of HIV-1 by hydroxyurea was irreversible, even several weeks after suspension of drug treatment. The indirect inhibition of HIV-1 by hydroxyurea is not expected to generate high rates of escape mutants. Hydroxyurea therefore appears to be a possible candidate for AIDS therapy.
PMID:7973634 Lori F et al; Science 266 (5186): 801:5 (1994)
Hydroxyurea (HU) is effectively used in the management of beta-hemoglobinopathies by augmenting the production of fetal hemoglobin (HbF). However, the molecular mechanisms underlying HU-mediated HbF regulation remain unclear. We previously reported that overexpression of the HU-induced SAR1 gene closely mimics the known effects of HU on K562 and CD34(+) cells, including gamma-globin induction and cell-cycle regulation. Here, we show that HU stimulated nuclear factor-kB interaction with its cognate-binding site on the SAR1 promoter to regulate transcriptional expression of SAR1 in K562 and CD34(+) cells. Silencing SAR1 expression not only significantly lowered both basal and HU-elicited HbF production in K562 and CD34(+) cells, but also significantly reduced HU-mediated S-phase cell-cycle arrest and apoptosis in K562 cells. Inhibition of c-Jun N-terminal kinase (JNK)/Jun phosphorylation and silencing of Gia expression in SAR1-transfected K562 and CD34(+) cells reduced both gamma-globin expression and HbF level, indicating that activation of Gia/JNK/Jun proteins is required for SAR1-mediated HbF induction. Furthermore, reciprocal coimmunoprecipitation assays revealed an association between forcibly expressed SAR1 and Gia2 or Gia3 proteins in both K562 and nonerythroid cells. These results indicate that HU induces SAR1, which in turn activates gamma-globin expression, predominantly through the Gia/JNK/Jun pathway. Our findings identify SAR1 as an alternative therapeutic target for beta-globin disorders.
PMID:24914133 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4133487 Zhu J et al; Blood 124 (7): 1146-56 (2014)
Hydroxyurea is well absorbed after oral administration, converted to a free radical nitroxide in vivo, and transported by diffusion into cells where it quenches the tyrosyl free radical at the active site of the M2 protein subunit of ribonucleotide reductase, inactivating the enzyme. The entire replitase complex, including ribonucleotide reductase, is inactivated and DNA synthesis is selectively inhibited, producing cell death in S phase and synchronization of the fraction of cells that survive. Repair of DNA damaged by chemicals or irradiation is also inhibited by hydroxyurea, offering potential synergy between hydroxyurea and radiation or alkylating agents. Hydroxyurea renders cells sensitive to bleomycin because the quenched tyrosyl free radical no longer stabilizes the adjacent iron center, making it more susceptible to the chelating properties of bleomycin, which then produces active oxygen. Synergy has also been observed between hydroxyurea and a number of other chemotherapeutic agents, including cytarabine and etoposide. Recently, two new effects of hydroxyurea have been observed: hydroxyurea increases the level of fetal hemoglobin, leading to a reduction in the incidence of vasoocclusive crises in sickle cell anemia, and hydroxyurea selectively reduces the level of episomal DNA and thus potentially may reduce drug resistance associated with duplicated genes retained as episomes.
Yarbro JW; Semin Oncol 19 (3 Suppl 9): 1-10 (1992)
Fanconi's anemia (FA) is a recessive disease; 16 genes are currently recognized in FA. FA proteins participate in the FA/BRCA pathway that plays a crucial role in the repair of DNA damage induced by crosslinking compounds. Hydroxyurea (HU) is an agent that induces replicative stress by inhibiting ribonucleotide reductase (RNR), which synthesizes deoxyribonucleotide triphosphates (dNTPs) necessary for DNA replication and repair. HU is known to activate the FA pathway; however, its clastogenic effects are not well characterized. We have investigated the effects of HU treatment alone or in sequential combination with mitomycin-C (MMC) on FA patient-derived lymphoblastoid cell lines from groups FA-A, B, C, D1/BRCA2, and E and on lymphocytes from two unclassified FA patients. All FA cells showed a significant increase (P < 0.05) in chromosomal aberrations following treatment with HU during the last 3 h before mitosis. Furthermore, when FA cells previously exposed to MMC were treated with HU, we observed an increase of MMC-induced DNA damage that was characterized by high occurrence of DNA breaks and a reduction in rejoined chromosomal aberrations. These findings show that exposure to HU during G2 induces chromosomal aberrations by a mechanism that is independent of its well-known role in replication fork stalling during S-phase and that HU interfered mainly with the rejoining process of DNA damage. We suggest that impaired oxidative stress response, lack of an adequate amount of dNTPs for DNA repair due to RNR inhibition, and interference with cell cycle control checkpoints underlie the clastogenic activity of HU in FA cells.
PMID:25663157 Molina B et al; Environ Mol Mutagen. 2015 Feb 6. doi: 10.1002/em.21938. (Epub ahead of print)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
13
PharmaCompass offers a list of Hydroxyurea API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Hydroxyurea manufacturer or Hydroxyurea supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Hydroxyurea manufacturer or Hydroxyurea supplier.
PharmaCompass also assists you with knowing the Hydroxyurea API Price utilized in the formulation of products. Hydroxyurea API Price is not always fixed or binding as the Hydroxyurea Price is obtained through a variety of data sources. The Hydroxyurea Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Hydroxyurea manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Hydroxyurea, including repackagers and relabelers. The FDA regulates Hydroxyurea manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Hydroxyurea API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Hydroxyurea manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Hydroxyurea supplier is an individual or a company that provides Hydroxyurea active pharmaceutical ingredient (API) or Hydroxyurea finished formulations upon request. The Hydroxyurea suppliers may include Hydroxyurea API manufacturers, exporters, distributors and traders.
click here to find a list of Hydroxyurea suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Hydroxyurea DMF (Drug Master File) is a document detailing the whole manufacturing process of Hydroxyurea active pharmaceutical ingredient (API) in detail. Different forms of Hydroxyurea DMFs exist exist since differing nations have different regulations, such as Hydroxyurea USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Hydroxyurea DMF submitted to regulatory agencies in the US is known as a USDMF. Hydroxyurea USDMF includes data on Hydroxyurea's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Hydroxyurea USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Hydroxyurea suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Hydroxyurea Drug Master File in Japan (Hydroxyurea JDMF) empowers Hydroxyurea API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Hydroxyurea JDMF during the approval evaluation for pharmaceutical products. At the time of Hydroxyurea JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Hydroxyurea suppliers with JDMF on PharmaCompass.
A Hydroxyurea CEP of the European Pharmacopoeia monograph is often referred to as a Hydroxyurea Certificate of Suitability (COS). The purpose of a Hydroxyurea CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Hydroxyurea EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Hydroxyurea to their clients by showing that a Hydroxyurea CEP has been issued for it. The manufacturer submits a Hydroxyurea CEP (COS) as part of the market authorization procedure, and it takes on the role of a Hydroxyurea CEP holder for the record. Additionally, the data presented in the Hydroxyurea CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Hydroxyurea DMF.
A Hydroxyurea CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Hydroxyurea CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Hydroxyurea suppliers with CEP (COS) on PharmaCompass.
A Hydroxyurea written confirmation (Hydroxyurea WC) is an official document issued by a regulatory agency to a Hydroxyurea manufacturer, verifying that the manufacturing facility of a Hydroxyurea active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Hydroxyurea APIs or Hydroxyurea finished pharmaceutical products to another nation, regulatory agencies frequently require a Hydroxyurea WC (written confirmation) as part of the regulatory process.
click here to find a list of Hydroxyurea suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Hydroxyurea as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Hydroxyurea API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Hydroxyurea as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Hydroxyurea and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Hydroxyurea NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Hydroxyurea suppliers with NDC on PharmaCompass.
Hydroxyurea Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Hydroxyurea GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Hydroxyurea GMP manufacturer or Hydroxyurea GMP API supplier for your needs.
A Hydroxyurea CoA (Certificate of Analysis) is a formal document that attests to Hydroxyurea's compliance with Hydroxyurea specifications and serves as a tool for batch-level quality control.
Hydroxyurea CoA mostly includes findings from lab analyses of a specific batch. For each Hydroxyurea CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Hydroxyurea may be tested according to a variety of international standards, such as European Pharmacopoeia (Hydroxyurea EP), Hydroxyurea JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Hydroxyurea USP).
