
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
VMF
DRUG PRODUCT COMPOSITIONS
0
Weekly News Recap #Phispers
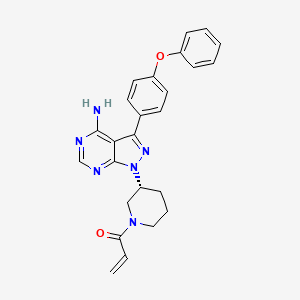

1. 1-((3r)-3-(4-amino-3-(4-phenoxyphenyl)-1h-pyrazolo(3,4-d)pyrimidin-1-yl)piperidin-1- Yl)prop-2-en-1-one
2. Imbruvica
3. Pci 32765
4. Pci-32765
5. Pci32765
1. 936563-96-1
2. Pci-32765
3. Imbruvica
4. Pci 32765
5. Ibrutinib (pci-32765)
6. Pci-32765 (ibrutinib)
7. Cra-032765
8. Pc-32765
9. (r)-1-(3-(4-amino-3-(4-phenoxyphenyl)-1h-pyrazolo[3,4-d]pyrimidin-1-yl)piperidin-1-yl)prop-2-en-1-one
10. 1x70osd4vx
11. Pci-32765-00
12. Chebi:76612
13. Pci32765
14. 1-[(3r)-3-[4-amino-3-(4-phenoxyphenyl)pyrazolo[3,4-d]pyrimidin-1-yl]piperidin-1-yl]prop-2-en-1-one
15. 1-((3r)-3-(4-amino-3-(4-phenoxyphenyl)-1h-pyrazolo(3,4-d)pyrimidin-1-yl)piperidin-1- Yl)prop-2-en-1-one
16. 1-[(3r)-3-[4-amino-3-(4-phenoxyphenyl)-1h-pyrazolo[3,4-d]pyrimidin-1-yl]piperidin-1-yl]prop-2-en-1-one
17. 1-{(3r)-3-[4-amino-3-(4-phenoxyphenyl)-1h-pyrazolo[3,4-d]pyrimidin-1-yl]piperidin-1-yl}prop-2-en-1-one
18. 2-propen-1-one, 1-((3r)-3-(4-amino-3-(4-phenoxyphenyl)-1h-pyrazolo(3,4-d)pyrimidin-1-yl)-1-piperidinyl)-
19. (r)-1-(3-(4-amino-3-(4-phenoxyphenyl)-1h-pyrazolo-[3,4-d]pyrimidin-1-yl)piperidin-1-yl)prop-2-en-1-one
20. (r)-1-(3-(4-amino-3-(4-phenoxyphenyl)-1h-pyrazolo[3,4-d]pyrimidin-1-yl)piperidin-1-yl)prop-2-en-1-one.
21. Ibrutinib [usan]
22. Ibrutinib [usan:inn]
23. Unii-1x70osd4vx
24. Ibrutinibum
25. Jnj 02
26. Imbruvica (tn)
27. Ibrutinib- Bio-x
28. Cra 032765
29. Ibrutinib [inn]
30. Ibrutinib [jan]
31. Ibrutinib [mi]
32. Ibrutinib (jan/usan)
33. Ibrutinib [vandf]
34. Ibrutinib [who-dd]
35. Imbruvica; Pci-32765
36. 1-[(3r)-3-[4-amino-3-(4-phenoxyphenyl)-1h-pyrazolo[3,4-d]pyrimidin-1-yl]-1-piperidinyl]-2-propen-1-one
37. Mls006010041
38. Schembl201859
39. Gtpl6912
40. Ibrutinib [orange Book]
41. Chembl1873475
42. Hsdb 8260
43. Dtxsid60893450
44. Ex-a066
45. Amy27873
46. Bdbm50357312
47. Mfcd20261150
48. Nsc800769
49. Zinc35328014
50. Akos022185476
51. Db09053
52. Ex-5960
53. Nsc-800769
54. Ncgc00187912-01
55. Ncgc00187912-02
56. Ncgc00187912-03
57. Ncgc00187912-12
58. Ac-26942
59. Bi164531
60. Hy-10997
61. Smr004701213
62. Sw218096-2
63. D10223
64. A1-01649
65. J-523872
66. Q5984881
67. (r)-3-(4-amino-3-(4-phenoxyphenyl)-1h-pyrazolo[3,4-d]pyrimidin-1-yl)piperidin-1-ylprop-2-en-1-one
68. 1-((r)-3-(4-amino-3-(4-phenoxyphenyl)-1h-pyrazolo[3,4-d]pyrimidin-1-yl)piperidin-1-yl)prop-2-en-1-one
69. 1-[(3r)-3-[4-amino-3-(4-phenoxyphenyl)-1h-pyrazolo[3,4-d]pyrimidin-1-yl]-1-piperidinyl]-2-propen-1-one1-[(3r)-3-[4-amino-3-(4-phenoxyphenyl)pyrazolo[3, 4-d]pyrimidin-1-yl]
70. 1-[(3r)-3-[4-amino-3-(4-phenoxyphenyl)pyrazolo[3, 4-d]pyrimidin-1-yl]piperidin-1-yl]prop-2-en-1-one
71. Ibrutinibci-32765mbruvica(r)-1-(3-(4-amino-3-(4-phenoxyphenyl)-1h-pyrazolo[3,4-d]pyrimidin-1-yl)piperidin-1-yl)prop-2-en-1-one
72. Piperidin-1-yl]prop-2-en-1-one1-{3-[4-amino-3-(4-phenoxy-phenyl)-pyrazolo[3,4-d]pyrimidin-1-yl]-piperidin-1-yl}-propenone
| Molecular Weight | 440.5 g/mol |
|---|---|
| Molecular Formula | C25H24N6O2 |
| XLogP3 | 3.6 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 5 |
| Exact Mass | 440.19607403 g/mol |
| Monoisotopic Mass | 440.19607403 g/mol |
| Topological Polar Surface Area | 99.2 Ų |
| Heavy Atom Count | 33 |
| Formal Charge | 0 |
| Complexity | 678 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Ibrutinib is a novel oral tyrosine kinase inhibitor that irreversibly binds and inhibits tyrosine-protein kinase BTK (Bruton tyrosine kinase). BTK has been found to be important in the function of B-cell receptor signaling and therefore in the maintenance and expansion of various B-cell malignancies including chronic lymphocytic leukemia (CLL) and mantle cell lymphoma (MCL). Targeting BTK with ibrutinib has been found to be an effective strategy in treating these malignancies. Phase I clinical testing in non-Hodgkin's lymphomas and CLL showed that the drug was extremely well tolerated with no major dose-limiting toxicities and a 54% overall response rate. Subsequently, two phase Ib/II studies were performed on patients with CLL, one in relapsed/refractory CLL and one in previously untreated elderly patients with CLL. Both of these studies continued to show good tolerability of the drug and an overall response rate of about 71% with extended duration of response. Another phase II study using ibrutinib in relapsed/refractory MCL was conducted and also showed that it was well tolerated with an overall response rate of 68% and extended duration of response. Due to these results, the U.S. Food and Drug Administration granted accelerated approval for ibrutinib in November 2013 for patients with MCL who had received at least one prior therapy and in February 2014 for patients with CLL who had received at least one prior therapy. This review will discuss the preclinical pharmacology, pharmacokinetics and clinical efficacy to date of ibrutinib in the treatment of CLL and MCL.
PMID:24918646 McDermott J, Jimeno A; Drugs Today (Barc) 50(4):291-300 (2014)
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health(NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Ibrutinib is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of July 18, 2015: https://clinicaltrials.gov/search/intervention=%22PCI-32765%22+OR+%22CRA-032765%22+OR+Ibrutinib+OR+%22PCI+32765%22+OR+PCI32765
Imbruvica is indicated for the treatment of patients with mantle cell lymphoma (MCL) who have received at least one prior therapy. Accelerated approval was granted for this indication based on overall response rate. Continued approval for this indication may be contingent upon verification of clinical benefit in confirmatory trials. /Included in US product label/
NIH; DailyMed. Current Medication Information for Imbruvica (Ibrutinib) Capsule (Updated: February 2015). Available from, as of May 4, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0dfd0279-ff17-4ea9-89be-9803c71bab44
Imbruvica is indicated for the treatment of patients with chronic lymphocytic leukemia (CLL) who have received at least one prior therapy. /Included in US product label/
NIH; DailyMed. Current Medication Information for Imbruvica (Ibrutinib) Capsule (Updated: February 2015). Available from, as of May 4, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0dfd0279-ff17-4ea9-89be-9803c71bab44
For more Therapeutic Uses (Complete) data for IBRUTINIB (7 total), please visit the HSDB record page.
Fatal and non-fatal infections have occurred with IMBRUVICA therapy. Grade 3 or greater infections occurred in 14% to 26% of patients. Cases of progressive multifocal leukoencephalopathy (PML) have occurred in patients treated with IMBRUVICA. Monitor patients for fever and infections and evaluate promptly.
NIH; DailyMed. Current Medication Information for Imbruvica (Ibrutinib) Capsule (Updated: February 2015). Available from, as of July 22, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0dfd0279-ff17-4ea9-89be-9803c71bab44#S1.4
Fatal bleeding events have occurred in patients treated with IMBRUVICA. Grade 3 or higher bleeding events (subdural hematoma, gastrointestinal bleeding, hematuria and post procedural hemorrhage) have occurred in up to 6% of patients. Bleeding events of any grade, including bruising and petechiae, occurred in approximately half of patients treated with IMBRUVICA. The mechanism for the bleeding events is not well understood. IMBRUVICA may increase the risk of hemorrhage in patients receiving antiplatelet or anticoagulant therapies. Consider the benefit-risk of withholding IMBRUVICA for at least 3 to 7 days pre and post-surgery depending upon the type of surgery and the risk of bleeding.
NIH; DailyMed. Current Medication Information for Imbruvica (Ibrutinib) Capsule (Updated: February 2015). Available from, as of July 20, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0dfd0279-ff17-4ea9-89be-9803c71bab44#S1.4
Treatment-emergent Grade 3 or 4 cytopenias including neutropenia (range, 19 to 29%), thrombocytopenia (range, 5 to 17%), and anemia (range, 0 to 9%) occurred in patients treated with IMBRUVICA. Monitor complete blood counts monthly.
NIH; DailyMed. Current Medication Information for Imbruvica (Ibrutinib) Capsule (Updated: February 2015). Available from, as of July 22, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0dfd0279-ff17-4ea9-89be-9803c71bab44#S1.4
Atrial fibrillation and atrial flutter (range, 6 to 9%) have occurred in patients treated with IMBRUVICA, particularly in patients with cardiac risk factors, acute infections, and a previous history of atrial fibrillation. Periodically monitor patients clinically for atrial fibrillation. Patients who develop arrhythmic symptoms (e.g., palpitations, lightheadedness) or new onset dyspnea should have an ECG performed. If atrial fibrillation persists, consider the risks and benefits of IMBRUVICA treatment and dose modification.
NIH; DailyMed. Current Medication Information for Imbruvica (Ibrutinib) Capsule (Updated: February 2015). Available from, as of July 22, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0dfd0279-ff17-4ea9-89be-9803c71bab44#S1.4
For more Drug Warnings (Complete) data for IBRUTINIB (8 total), please visit the HSDB record page.
Ibrutinib acquired an accelerated approval for the treatment of mantle cell lymphoma who have received at least one prior therapy. Mantle cell lymphoma (MCL) is a B-cell non-Hodgkin lymphoma that develops in the outer edge of a lymph node. MCL is usually diagnosed at late stages and it is easily spread into bone marrow, spleen, liver and gastrointestinal tract. Ibrutinib is indicated for the treatment of chronic lymphocytic leukemia (CLL) who have at least one prior therapy. CLL is a type of cancer caused by an overproduction of lymphocytes by the bone marrow. Some of the symptoms include swollen lymph nodes and tiredness. Ibrutinib is indicated for the treatment of chronic lymphocytic leukemia (CLL) with 17p deletion. CLL with 17p is a type of leukemia in which a deletion in 17p disrupts the tumor suppressor p53 by deleting one allele of the TP53 gene. The remaining allele is mainly inactivated and thus, this type of leukemia is unresponsive to p53-dependent treatments. Ibrutinib is indicated for the treatment of patients with Waldenstrom's Macroglobulinemia (WM). WM, also called lymphoplasmacytic lymphoma, is a type of non-Hodgkin lymphoma in which the cancer cells make large amounts of macroglobulin. The macroglobulin is a monoclonal protein that corresponds to the type of IgM antibodies and the unrestricted formation of this protein causes typical symptoms such as excessive bleeding and effects in vision and nervous system.
FDA Label
IMBRUVICA as a single agent is indicated for the treatment of adult patients with relapsed or refractory mantle cell lymphoma (MCL).
IMBRUVICA as a single agent or in combination with rituximab or obinutuzumab or venetoclax is indicated for the treatment of adult patients with previously untreated chronic lymphocytic leukaemia (CLL) (see section 5. 1).
IMBRUVICA as a single agent or in combination with bendamustine and rituximab (BR) is indicated for the treatment of adult patients with CLL who have received at least one prior therapy.
IMBRUVICA as a single agent is indicated for the treatment of adult patients with Waldenstrms macroglobulinaemia (WM) who have received at least one prior therapy, or in first line treatment for patients unsuitable for chemo immunotherapy. IMBRUVICA in combination with rituximab is indicated for the treatment of adult patients with WM.
Treatment of chronic Graft versus Host Disease (cGvHD)
Treatment of lymphoplasmacytic lymphoma
Treatment of mantle cell lymphoma
Treatment of lymphoplasmacytic lymphoma
Treatment of mantle cell lymphoma
Treatment of mature B-cell neoplasms
In vitro studies have shown an induction of CLL cell apoptosis even in presence of prosurvival factors. It has also been reported an inhibition of CLL cell survival and proliferation as well as an impaired in cell migration and a reduction in the secretion of chemokines such as CCL3 and CCL4. The latter effect has been shown to produce regression in xenograft mouse models. Clinical studies for relapsed/refractory CLL in phase I and II showed an approximate 71% of overall response rate.. In the case of relapsed/refractory mantle cell lymphoma, approximately 70% of the tested patients presented a partial or complete response.. In clinical trials for relapsed/refractory diffuse large B-cell lymphoma, a partial response was found in between 15-20% of the patients studied; while for patients with relapsed/refractory Waldenstrom's macroglobulinemia, a partial response was observed in over 75% of the patients tested. Finally, for patients with relapsed/refractory follicular lymphoma, a partial to complete response was obtained in approximately 54% of the patients.
L01EL01
L01XE27
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01E - Protein kinase inhibitors
L01EL - Bruton's tyrosine kinase (btk) inhibitors
L01EL01 - Ibrutinib
Absorption
Ibrutinib is rapidly absorbed after oral administration and it presents a Cmax, tmax and AUC of approximately 35 ng/ml, 1-2 hour and 953 mg.h/ml respectively.
Route of Elimination
The cumulative excretion of ibrutinib in urine is of about 7.8% of the administered dose and most of this excretion is found during the first 24 hours after administration. In feces, the cumulative excretion accounts for 80% of the administered dose and the excretion occurs within 48 hours of the initial administration. The total excretion of ibrutinib during the first 168 hours after initial administration accounts for 88.5% of the administered dose.
Volume of Distribution
The volume of distribution at steady-state of ibrutinib is in approximately 10,000 L.
Clearance
In patients with normal renal function, the clearance rate is in the range of 112-159 ml/min.
Three metabolic pathways have been identified according to the possible metabolites. These pathways are the hydroxylation of the phenyl group (M35), the opening of the piperidine with a reduction of the primary alcohol (M34) and the oxidation to a carboxylic acid and epoxidation of the ethylene followed by a hydrolysis to the formation of dihydrodiol (PCI-45227). The latter metabolite presents also 15 times lower inhibitory activity against BTK. The metabolism of ibrutinib is mainly performed by CYP3A5 and CYP3A4. and in a minor extent it is seen to be performed by CYP2D6.
Since 2014, Ibrutinib has been available as a new drug for the treatment of leukemic diseases. Ibrutinib (Imbruvica) is metabolized in the liver mainly by the isoenzyme CYP3A4 and to a minor extent by CYP2D6. Simultaneous application of Imbruvica and consumption of foods containing secondary metabolites strongly inhibiting the CYP3A4 isoform, could significantly influence the toxicity of this drug. This article references the respective foods.
PMID:25975016 Kronabel D; Clin Lab 61 (3-4): 443-4 (2015)
The elimination half-life of ibrutinib is of approximately 4-6 hours.
Ibrutinib is an inhibitor of Brutons tyrosine kinase (BTK). It forms a covalent bond with a cysteine residue in the active site of BTK (Cys481), leading to its inhibition. The inhibition of BTK plays a role in the B-cell receptor signaling and thus, the presence of ibrutinib prevents the phosphorylation of downstream substrates such as PLC-.
Ibrutinib is a novel oral tyrosine kinase inhibitor that irreversibly binds and inhibits tyrosine-protein kinase BTK (Bruton tyrosine kinase). BTK has been found to be important in the function of B-cell receptor signaling and therefore in the maintenance and expansion of various B-cell malignancies including chronic lymphocytic leukemia (CLL) and mantle cell lymphoma (MCL). Targeting BTK with ibrutinib has been found to be an effective strategy in treating these malignancies. Phase I clinical testing in non-Hodgkin's lymphomas and CLL showed that the drug was extremely well tolerated with no major dose-limiting toxicities and a 54% overall response rate. Subsequently, two phase Ib/II studies were performed on patients with CLL, one in relapsed/refractory CLL and one in previously untreated elderly patients with CLL. Both of these studies continued to show good tolerability of the drug and an overall response rate of about 71% with extended duration of response. Another phase II study using ibrutinib in relapsed/refractory MCL was conducted and also showed that it was well tolerated with an overall response rate of 68% and extended duration of response. Due to these results, the U.S. Food and Drug Administration granted accelerated approval for ibrutinib in November 2013 for patients with MCL who had received at least one prior therapy and in February 2014 for patients with CLL who had received at least one prior therapy. This review will discuss the preclinical pharmacology, pharmacokinetics and clinical efficacy to date of ibrutinib in the treatment of CLL and MCL.
PMID:24918646 McDermott J, Jimeno A; Drugs Today (Barc) 50(4):291-300 (2014)
... In this study we report for the first time that ibrutinib is cytotoxic to malignant plasma cells from patients with multiple myeloma (MM) and furthermore that treatment with ibrutinib significantly augments the cytotoxic activity of bortezomib and lenalidomide chemotherapies. We describe that the cytotoxicity of ibrutinib in MM is mediated via an inhibitory effect on the nuclear factor-(k)B (NF-(k)B) pathway. Specifically, ibrutinib blocks the phosphorylation of serine-536 of the p65 subunit of NF-(k)B, preventing its nuclear translocation, resulting in down-regulation of anti-apoptotic proteins Bcl-xL, FLIP(L) and survivin and culminating in caspase-mediated apoptosis within the malignant plasma cells....
PMID:22975686 Rushworth SA et al; Cell Signal 25 (1): 106-12 (2013)
Mantle cell lymphoma (MCL) is an aggressive B-cell malignancy that characteristically shows overexpression of cyclin-D1 due to an alteration in the t(11;14)(q13;q32) chromosomal region. Although there are some promising treatment modalities, great majority of patients with this disease remain incurable. The B-cell antigen receptor (BCR) signaling plays a crucial role in B-cell biology and lymphomagenesis. Bruton tyrosine kinase (BTK) has been identified as a key component of the BCR signaling pathway. Evidence suggests that the blockade of BTK activity by potent pharmacologic inhibitors attenuates BCR signaling and induces cell death. Notably, the expression levels and the role of BTK in MCL survival are still elusive. Here, we demonstrated a moderate to strong BTK expression in all MCL cases (n=19) compared to benign lymphoid tissues. Treatment of MCL cell lines (Mino or Jeko-1) with a potent BTK pharmacologic inhibitor, Ibrutinib, decreased phospho-BTK-Tyr(223) expression. Consistent with this observation, Ibrutinib inhibited the viability of both Mino and JeKo-1 cells in concentration- and time-dependent manners. Ibrutinib also induced a concentration-dependent apoptosis in both cell lines. Consistently, Ibrutinib treatment decreased the levels of anti-apoptotic Bcl-2, Bcl-xL, and Mcl-1 protein. These findings suggest that BTK signaling plays a critical role in MCL cell survival, and the targeting of BTK could represent a promising therapeutic modality for aggressive lymphoma.
PMID:23962569 Cinar M et al; Leuk Res 37 (10): 1271-7 (2013)
Ibrutinib is a small-molecule inhibitor of Bruton's tyrosine kinase (BTK). Ibrutinib forms a covalent bond with a cysteine residue in the BTK active site, leading to inhibition of BTK enzymatic activity. BTK is a signaling molecule of the B-cell antigen receptor (BCR) and cytokine receptor pathways. BTK's role in signaling through the B-cell surface receptors results in activation of pathways necessary for B-cell trafficking, chemotaxis, and adhesion. Nonclinical studies show that ibrutinib inhibits malignant B-cell proliferation and survival in vivo as well as cell migration and substrate adhesion in vitro.
NIH; DailyMed. Current Medication Information for Imbruvica (Ibrutinib) Capsule (Updated: February 2015). Available from, as of May 20, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0dfd0279-ff17-4ea9-89be-9803c71bab44

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
100
PharmaCompass offers a list of Ibrutinib API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Ibrutinib manufacturer or Ibrutinib supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Ibrutinib manufacturer or Ibrutinib supplier.
PharmaCompass also assists you with knowing the Ibrutinib API Price utilized in the formulation of products. Ibrutinib API Price is not always fixed or binding as the Ibrutinib Price is obtained through a variety of data sources. The Ibrutinib Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Ibrutinib manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Ibrutinib, including repackagers and relabelers. The FDA regulates Ibrutinib manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Ibrutinib API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Ibrutinib manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Ibrutinib supplier is an individual or a company that provides Ibrutinib active pharmaceutical ingredient (API) or Ibrutinib finished formulations upon request. The Ibrutinib suppliers may include Ibrutinib API manufacturers, exporters, distributors and traders.
click here to find a list of Ibrutinib suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Ibrutinib DMF (Drug Master File) is a document detailing the whole manufacturing process of Ibrutinib active pharmaceutical ingredient (API) in detail. Different forms of Ibrutinib DMFs exist exist since differing nations have different regulations, such as Ibrutinib USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Ibrutinib DMF submitted to regulatory agencies in the US is known as a USDMF. Ibrutinib USDMF includes data on Ibrutinib's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Ibrutinib USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Ibrutinib suppliers with USDMF on PharmaCompass.
A Ibrutinib written confirmation (Ibrutinib WC) is an official document issued by a regulatory agency to a Ibrutinib manufacturer, verifying that the manufacturing facility of a Ibrutinib active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Ibrutinib APIs or Ibrutinib finished pharmaceutical products to another nation, regulatory agencies frequently require a Ibrutinib WC (written confirmation) as part of the regulatory process.
click here to find a list of Ibrutinib suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Ibrutinib as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Ibrutinib API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Ibrutinib as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Ibrutinib and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Ibrutinib NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Ibrutinib suppliers with NDC on PharmaCompass.
Ibrutinib Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Ibrutinib GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Ibrutinib GMP manufacturer or Ibrutinib GMP API supplier for your needs.
A Ibrutinib CoA (Certificate of Analysis) is a formal document that attests to Ibrutinib's compliance with Ibrutinib specifications and serves as a tool for batch-level quality control.
Ibrutinib CoA mostly includes findings from lab analyses of a specific batch. For each Ibrutinib CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Ibrutinib may be tested according to a variety of international standards, such as European Pharmacopoeia (Ibrutinib EP), Ibrutinib JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Ibrutinib USP).
