
Synopsis
Synopsis
0
JDMF
0
KDMF
0
VMF
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
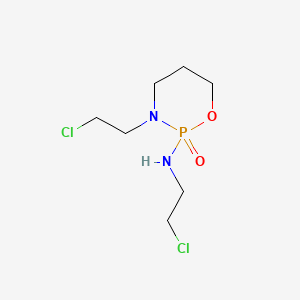

1. Asta Z 4942
2. Holoxan
3. Iphosphamide
4. Iso Endoxan
5. Iso-endoxan
6. Isofosfamide
7. Isophosphamide
8. Nsc 109,724
9. Nsc 109724
10. Nsc-109,724
11. Nsc-109724
12. Nsc109,724
13. Nsc109724
1. Isophosphamide
2. 3778-73-2
3. Iphosphamide
4. Isofosfamide
5. Ifex
6. Ifosfamid
7. Mitoxana
8. Iphosphamid
9. Isoendoxan
10. Naxamide
11. I-phosphamide
12. Holoxan
13. Cyfos
14. Ifsofamide
15. Holoxan 1000
16. Asta Z 4942
17. Mjf 9325
18. Ifosfamida
19. Ifosfamidum
20. Mjf-9325
21. Isosfamide
22. Nsc-109724
23. Nci-c01638
24. Z4942
25. Z-4942
26. Ifomide
27. Nsc 109724
28. A 4942
29. Z 4942
30. 2h-1,3,2-oxazaphosphorin-2-amine, N,3-bis(2-chloroethyl)tetrahydro-, 2-oxide
31. 3-(2-chloroethyl)-2-((2-chloroethyl)amino)tetrahydro-2h-1,3,2-oxazaphosphorine 2-oxide
32. Nsc109724
33. N,3-bis(2-chloroethyl)-1,3,2-oxazaphosphinan-2-amine 2-oxide
34. N-(2-chloroethyl)-n'-(2-chloroethyl)-n',o-propylenephosphoric Acid Diamide
35. Um20qqm95y
36. N,3-bis(2-chloroethyl)tetrahydro-2h-1,3,2-oxazaphosphorin-2-amine 2-oxide
37. 3-(2-chloroethyl)-2-((2-chloroethyl)amino)-1,3,2-oxazaphosphinane 2-oxide
38. Chebi:5864
39. Ifosphamide
40. 1,3,2-oxazaphosphorine, 3-(2-chloroethyl)-2-((2-chloroethyl)amino)tetrahydro-, 2-oxide
41. 2h-1,3,2-oxazaphosphorine, 3-(2-chloroethyl)-2-((2-chloroethyl)amino)tetrahydro-, 2-oxide
42. 3-(2-chloroethyl)-2-(2-chloroethylamino)tetrahydro-2h-1,3,2-oxaazaphosphorin 2-oxide
43. 3-(2-chloroethyl)-2-[(2-chloroethyl)amino]perhydro-2h-1,3,2-oxazaphosphorineoxide
44. N-(2-chloroethyl)-n'-(2-chloroethyl)-n',o-propylene Phosphoric Acid Ester Diamide
45. Ncgc00016639-01
46. Cas-3778-73-2
47. Dsstox_cid_760
48. Ifosfamide Sterile
49. Dsstox_rid_75775
50. Dsstox_gsid_20760
51. Ifosfamidum [inn-latin]
52. Ifosfamida [inn-spanish]
53. (r)-ifosfamide
54. (s)-ifosfamide
55. (r)-3-(2-chloroethyl)-2-((2-chloroethyl)amino)-1,3,2-oxazaphosphinane 2-oxide
56. Ccris 352
57. Hsdb 7023
58. Sr-05000002022
59. Einecs 223-237-3
60. Ifex (tn)
61. Unii-um20qqm95y
62. Brn 0611835
63. Ifosfamide (jan/usp/inn)
64. N,3-bis(2-chloroethyl)-2-oxo-1,3,2?^{5}-oxazaphosphinan-2-amine
65. Ifosfamid A
66. 3-(2-chloroethyl)-2-((2-chloroethyl)amino)perhydro-2h-1,3,2-oxazaphosphorineoxide
67. Ifosfamide In Bulk
68. 2,3-(n,n(sup 1)-bis(2-chloroethyl)diamido)-1,3,2-oxazaphosphoridinoxyd
69. Isophosphamide,(s)
70. N,n-bis(beta-chloroethyl)-amino-n',o-propylene-phosphoric Acid Ester Diamide
71. N-(2-chloroethyl)-n'-(2-chloroethyl)-n',o-propylenephosphoric Acid Ester Diamide
72. Mfcd00057374
73. 3-(2-chloroethyl)-2-((2-chloroethyl)amino)perhydro-2h-1,3,2-oxazaphosphorine Oxide
74. N-(2-chloraethyl)-n'-(2-chloraethyl)-n',o-propylen-phosphorsaureester-diamid [german]
75. Ifosfamide - Bio-x
76. Ifosfamide [usan:usp:inn:ban:jan]
77. Starbld0001221
78. Ifosfamide, >=98%
79. Ifosfamide [mi]
80. Isocyclophosphamide
81. Ifosfamide [inn]
82. Ifosfamide [jan]
83. Prestwick0_000833
84. Prestwick1_000833
85. Prestwick2_000833
86. Prestwick3_000833
87. Ifosfamide [hsdb]
88. Ifosfamide [usan]
89. Intermediate Of Ifosfamide
90. Ifosfamide [vandf]
91. N-(2-chloraethyl)-n'-(2-chloraethyl)-n',o-propylen-phosphorsaureester-diamid
92. Ifosfamide [mart.]
93. Schembl4885
94. Chembl1024
95. Ifosfamide [usp-rs]
96. Ifosfamide [who-dd]
97. Bspbio_000785
98. Isophosphamide [iarc]
99. Mls002154021
100. Ifex (tn) (bristol Meyers)
101. Spbio_002706
102. Bpbio1_000865
103. Gtpl7201
104. Dtxsid7020760
105. Ifosfamide [ep Impurity]
106. Ifosfamide [orange Book]
107. Ifosfamide [ep Monograph]
108. (s)-3-(2-chloroethyl)-2-((2-chloroethyl)amino)-1,3,2-oxazaphosphinane 2-oxide
109. Bdbm189358
110. Hms1570h07
111. Hms2090m12
112. Hms2093n07
113. Hms2097h07
114. Hms2232o10
115. Hms3374b08
116. Hms3654b15
117. Hms3714h07
118. Ifosfamide [usp Monograph]
119. Pharmakon1600-01505480
120. {3-(2-chloroethyl)-2-[(2-
121. Bcp06596
122. Wln: T6npotj Am2g Bo B2g
123. Tox21_110539
124. Tox21_201815
125. Tox21_302775
126. Bbl028071
127. N,3-bis(2-chloroethyl)-2-oxo-1,3,2$l^{5}-oxazaphosphinan-2-amine
128. N,3-bis(2-chloroethyl)-2-oxo-1,3,2lambda5-oxazaphosphinan-2-amine
129. Nsc759154
130. S1302
131. Stl058690
132. Akos005711213
133. N,3-bis(2-chloroethyl)tetrahydro-2h-1,3,2-oxazaphosphorin-2-amine-2-oxide
134. Tox21_110539_1
135. Ab02316
136. Ac-2113
137. Ccg-213464
138. Cs-1424
139. Db01181
140. Nsc-759154
141. N-(2-chloroethyl)-n-(3-(2-chloroethyl)-2-oxido-1,3,2-oxazaphosphinan-2-yl)amine
142. Ifosfamide, Analytical Reference Material
143. Ncgc00179435-01
144. Ncgc00179435-02
145. Ncgc00179435-03
146. Ncgc00179435-06
147. Ncgc00179435-07
148. Ncgc00256413-01
149. Ncgc00259364-01
150. As-10978
151. Bi166243
152. Hy-17419
153. Nci60_000233
154. Smr001233348
155. Sbi-0206804.p001
156. Db-049196
157. Ab00513932
158. Ft-0603650
159. Ft-0670282
160. I0713
161. Sw197177-4
162. C07047
163. D00343
164. Ab00513932-06
165. Ab00513932-07
166. Ab00513932-08
167. Ab00513932_09
168. Ab00513932_10
169. 778i732
170. A823873
171. Q418560
172. Q-101874
173. Sr-05000002022-1
174. Sr-05000002022-3
175. Sr-05000002022-5
176. Brd-a67097164-001-11-2
177. Ifosfamide, British Pharmacopoeia (bp) Reference Standard
178. Ifosfamide, European Pharmacopoeia (ep) Reference Standard
179. Ifosfamide, United States Pharmacopeia (usp) Reference Standard
180. 2,n(sup 1)-bis(2-chloroethyl)diamido-1,3,2-oxazaphosphoridinoxy-
181. 2,n(sup 1)-bis(2-chloroethyl)diamido-1,3,2-oxazaphosphoridinoxyd
182. N,3-bis(2-chloroethyl)-2-oxo-1,3,2$l;{5}-oxazaphosphinan-2-amine
183. {3-(2-chloroethyl)-2-[(2-chloroethyl)amino]perhydro-2h-1,3,} 2-oxazaphosphorine Oxide
184. 1,2-oxazaphosphorine, 3-(2-chloroethyl)-2-[(2-chloroethyl)amino]tetrahydro-, 2-oxide
185. 2h-1,2-oxazaphosphorin-2-amine, N,3-bis(2-chloroethyl)tetrahydro-, 2-oxide
186. 2h-1,2-oxazaphosphorine, 3-(2-chloroethyl)-2-[(2-chloroethyl)amino]tetrahydro-, 2-oxide
187. 3-(2-chloroethyl)-2-(2-chloroethylamino)tetrahydro-2h-1,3,2-oxazaphosphorin-2-one
188. 3-(2-chloroethyl)-2-[(2-chloroethyl)amino]-1,3,2$l^{5}-oxazaphosphinan-2-one
189. 3-(2-chloroethyl)-2-[(2-chloroethyl)amino]-1,3,2lambda5-oxazaphosphinan-2-one
190. 3-(2-chloroethyl)-2-[(2-chloroethyl)amino]perhydro-2h-1,2-oxazaphosphorine Oxide
191. 3-(2-chloroethyl)-2-[(2-chloroethyl)amino]perhydro-2h-1,2-oxazaphosphorineoxide
192. 3-(2-chloroethyl)-2-[(2-chloroethyl)amino]tetrahydro-2h-1,2-oxazaphosphorine 2-oxide
193. N,3-bis(2-chloroethyl)tetrahydro-2h-1,3,2-oxazaphosphinan-2-amine 2-oxide #
| Molecular Weight | 261.08 g/mol |
|---|---|
| Molecular Formula | C7H15Cl2N2O2P |
| XLogP3 | 0.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 5 |
| Exact Mass | 260.0248201 g/mol |
| Monoisotopic Mass | 260.0248201 g/mol |
| Topological Polar Surface Area | 41.6 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 218 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Ifex |
| PubMed Health | Ifosfamide (Injection) |
| Drug Classes | Antineoplastic Agent |
| Drug Label | IFEX (ifosfamide for injection, USP) single-dose vials for constitution and administration by intravenous infusion each contain 1 gram or 3 grams of sterile ifosfamide. Ifosfamide is a chemotherapeutic agent chemically related to the nitrogen mustard... |
| Active Ingredient | Ifosfamide |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 1gm/vial; 3gm/vial |
| Market Status | Prescription |
| Company | Baxter Hlthcare |
| 2 of 4 | |
|---|---|
| Drug Name | Ifosfamide |
| PubMed Health | Ifosfamide (Injection) |
| Drug Classes | Antineoplastic Agent |
| Drug Label | Ifosfamide injection sterile, single-dose vials for administration by intravenous infusion each contains 1 gram or 3 grams of ifosfamide, USP. The 1-gram vial also contains 69.0 mg monobasic sodium phosphate monohydrate, USP, 21.3 mg dibasic sodium p... |
| Active Ingredient | Ifosfamide |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 1gm/20ml (50mg/ml); 3gm/60ml(50mg/ml); 1gm/vial; 3gm/vial; 1gm/20ml(50mg/ml); 3gm/60ml (50mg/ml) |
| Market Status | Prescription |
| Company | Teva Pharms Usa; Fresenius Kabi Usa; Onco Therapies; Eurohlth Intl |
| 3 of 4 | |
|---|---|
| Drug Name | Ifex |
| PubMed Health | Ifosfamide (Injection) |
| Drug Classes | Antineoplastic Agent |
| Drug Label | IFEX (ifosfamide for injection, USP) single-dose vials for constitution and administration by intravenous infusion each contain 1 gram or 3 grams of sterile ifosfamide. Ifosfamide is a chemotherapeutic agent chemically related to the nitrogen mustard... |
| Active Ingredient | Ifosfamide |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 1gm/vial; 3gm/vial |
| Market Status | Prescription |
| Company | Baxter Hlthcare |
| 4 of 4 | |
|---|---|
| Drug Name | Ifosfamide |
| PubMed Health | Ifosfamide (Injection) |
| Drug Classes | Antineoplastic Agent |
| Drug Label | Ifosfamide injection sterile, single-dose vials for administration by intravenous infusion each contains 1 gram or 3 grams of ifosfamide, USP. The 1-gram vial also contains 69.0 mg monobasic sodium phosphate monohydrate, USP, 21.3 mg dibasic sodium p... |
| Active Ingredient | Ifosfamide |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 1gm/20ml (50mg/ml); 3gm/60ml(50mg/ml); 1gm/vial; 3gm/vial; 1gm/20ml(50mg/ml); 3gm/60ml (50mg/ml) |
| Market Status | Prescription |
| Company | Teva Pharms Usa; Fresenius Kabi Usa; Onco Therapies; Eurohlth Intl |
Ifosfamide currently is approved for use in combination with other drugs for germ cell testicular cancer & is widely used to treat pediatric & adult sarcomas. Clinical trials also have shown ifosfamide to be active against carcinomas of the cervix & lung & against lymphomas. It is a common component of high-dose chemotherapy regimens with bone marrow or stem cell rescue; in these regimens, in total doses of 12-14 g/sq m, it may cause severe neurological toxicity, including coma & death. This toxicity is thought to result form a metabolite, chloracetaldehyde. In addition to hemorrhagic cystitis, ifosfamide causes nausea, vomiting, anorexia, leukopenia, nephrotoxicity, & CNS disturbances (especially somnolence & confusion).
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1396
Ifosfamide is indicated, in combination with other antineoplastic agents and a prophylactic agent against hemorrhagic cystitis (such as mesna), for treatment of germ cell testicular tumors. /Included in US product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1677
Ifosfamide is indicated as reasonable medical therapy for treatment of head and neck carcinoma. (Evidence rating: IIID) /NOT included in US product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1677
Ifosfamide is used for treatment of soft-tissue sarcomas, Ewing's sarcoma, and Hodgkin's and non-Hodgkin's lymphomas. /NOT included in US product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1677
For more Therapeutic Uses (Complete) data for IFOSFAMIDE (9 total), please visit the HSDB record page.
It is a common component of high-dose chemotherapy regimens with bone marrow or stem cell rescue; in these regimens, in total doses of 12-14 g/sq m, it may cause severe neurological toxicity, including coma & death. This toxicity is thought to result form a metabolite, chloracetaldehyde. In addition to hemorrhagic cystitis, ifosfamide causes nausea, vomiting, anorexia, leukopenia, nephrotoxicity, & CNS disturbances (especially somnolence & confusion).
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1396
Ifosfamide is distributed into breast milk. Breast feeding is not recommended during chemotherapy because of the risks to the infant (adverse effects, mutagenicity, carcinogenicity).
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1677
The bone marrow depressant effects of ifosfamide may result in an increased incidence of microbial infection, delayed healing, and gingival bleeding. Dental work, whenever possible, should be completed prior to initiation of therapy or deferred until blood counts have returned to normal. Patients should be instructed in proper oral hygiene during treatment, including caution in use of regular toothbrushes, dental floss, and toothpicks.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1677
Many side effects of antineoplastic therapy are unavoidable and represent the medication's pharmacologic action. Some of these (for example, leukopenia and thrombocytopenia) are actually used as parameters to aid in individual dosage titration.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 1678
For more Drug Warnings (Complete) data for IFOSFAMIDE (20 total), please visit the HSDB record page.
Used as a component of various chemotherapeutic regimens as third-line therapy for recurrent or refractory germ cell testicular cancer. Also used as a component of various chemotherapeutic regimens for the treatment of cervical cancer, as well as in conjunction with surgery and/or radiation therapy in the treatment of various soft tissue sarcomas. Other indications include treatment of osteosarcoma, bladder cancer, ovarian cancer. small cell lung cancer, and non-Hodgkin's lymphoma.
Ifosfamide requires activation by microsomal liver enzymes to active metabolites in order to exert its cytotoxic effects. Activation occurs by hydroxylation at the ring carbon atom 4 to form the unstable intermediate 4-hydroxyifosfamide. This metabolite than rapidly degrades to the stable urinary metabolite 4-ketoifosfamide. The stable urinary metabolite, 4-carboxyifosfamide, is formed upon opening of the ring. These urinary metabolites have not been found to be cytotoxic. N, N-bis (2-chloroethyl)-phosphoric acid diamide (ifosphoramide) and acrolein are also found. The major urinary metabolites, dechloroethyl ifosfamide and dechloroethyl cyclophosphamide, are formed upon enzymatic oxidation of the chloroethyl side chains and subsequent dealkylation. It is the alkylated metabolites of ifosfamide that have been shown to interact with DNA. Ifosfamide is cycle-phase nonspecific.
Antineoplastic Agents, Alkylating
A class of drugs that differs from other alkylating agents used clinically in that they are monofunctional and thus unable to cross-link cellular macromolecules. Among their common properties are a requirement for metabolic activation to intermediates with antitumor efficacy and the presence in their chemical structures of N-methyl groups, that after metabolism, can covalently modify cellular DNA. The precise mechanisms by which each of these drugs acts to kill tumor cells are not completely understood. (From AMA, Drug Evaluations Annual, 1994, p2026) (See all compounds classified as Antineoplastic Agents, Alkylating.)
L01AA06
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01A - Alkylating agents
L01AA - Nitrogen mustard analogues
L01AA06 - Ifosfamide
Route of Elimination
Ifosfamide is extensively metabolized in humans and the metabolic pathways appear to be saturated at high doses. After administration of doses of 5 g/m2 of 14C-labeled ifosfamide, from 70% to 86% of the dosed radioactivity was recovered in the urine, with about 61% of the dose excreted as parent compound. At doses of 1.62.4 g/m2 only 12% to 18% of the dose was excreted in the urine as unchanged drug within 72 hours.
Volume of Distribution
Ifosfamide volume of distribution (Vd) approximates the total body water volume, suggesting that distribution takes place with minimal tissue binding. Following intravenous administration of 1.5 g/m2 over 0.5 hour once daily for 5 days to 15 patients with neoplastic disease, the median Vd of ifosfamide was 0.64 L/kg on Day 1 and 0.72 L/kg on Day 5. When given to pediatric patients, the volume of distribution was 211.6 L/m^2.
Clearance
2.40.33 L/h/m^2 [pediatric patients]
Renal excretion & t1/2 are dose & schedule dependent. 60-80% recovered as unchanged drug or metabolite in urine within 72 hr after admin.
Young, L.Y., M.A. Koda-Kimble (eds.). Applied Therapeutics. The Clinical Use of Drugs. 6th ed. Vancouver, WA., Applied Therapeutics, Inc. 1995., p. 90-13
The distribution of ifosfamide (IF) and its metabolites 2-dechloroethylifosfamide (2DCE), 3-dechloroethylifosfamide (3DCE), 4-hydroxyifosfamide (4OHIF) and ifosforamide mustard (IFM) between plasma and erythrocytes was examined in vitro and in vivo. In vitro distribution was investigated by incubating blood with various concentrations of IF and its metabolites. In vivo distribution of IF, 2DCE, 3DCE and 4OHIF was determined in 7 patients receiving 9 g/m(2)/72 h intravenous continuous IF infusion. In vitro distribution equilibrium between erythrocytes and plasma was obtained quickly after drug addition. Mean (+/-sem) in vitro and in vivo erythrocyte (e)-plasma (p) partition coefficients (P(e/p)) were 0.75+/-0.01 and 0.81+/-0.03, 0.62+/-0.09 and 0.73+/-0.05, 0.76+/-0.10 and 0.93+/-0.05 and 1.38+/-0.04 and 0.98+/-0.09 for IF, 2DCE, 3DCE and 4OHIF, respectively. These ratios were independent of concentration and unaltered with time. The ratios of the area under the erythrocyte and plasma concentration--time curves (AUC(e/p)) were 0.96+/-0.03, 0.87+/-0.07, 0.98+/-0.06 and 1.34+/-0.39, respectively. A time- and concentration-dependent distribution--equilibrium phenomenon was observed with the relative hydrophilic IFM. It is concluded that IF and metabolites rapidly reach distribution equilibrium between erythrocytes and plasma; the process is slower for IFM. Drug distribution to the erythrocyte fraction ranged from about 38% for 2DCE to 58% for 4OHIF, and was stable over a wide range of clinically relevant concentrations. A strong parallelism in the erythrocyte and plasma concentration profiles was observed for all compounds. Thus, pharmacokinetic assessment using only plasma sampling yields direct and accurate insights into the whole blood kinetics of IF and metabolites and may be used for pharmacokinetic-pharmacodynamic studies.
PMID:11745912 Kerbusch T, et al; Biopharm Drug Dispos 22 (3): 99-108 (2001)
... To assess the feasibility of a sparse sampling approach for the determination of the population pharmacokinetics of ifosfamide, 2- and 3-dechloroethyl-ifosfamide and 4-hydroxy-ifosfamide in children treated with single-agent ifosfamide against various malignant tumours. ... Pharmacokinetic assessment followed by model fitting. Patients: The analysis included 32 patients aged between 1 and 18 years receiving a total of 45 courses of ifosfamide 1.2, 2 or 3 g/m2 in 1 or 3 hours on 1, 2 or 3 days. ... A total of 133 blood samples (median of 3 per patient) were collected. Plasma concentrations of ifosfamide and its dechloroethylated metabolites were determined by gas chromatography. Plasma concentrations of 4-hydroxy-ifosfamide were measured by high-performance liquid chromatography. The models were fitted to the data using a nonlinear mixed effects model as implemented in the NONMEM program. A cross-validation was performed. ... Population values (mean +/- standard error) for the initial clearance and volume of distribution of ifosfamide were estimated at 2.36 +/- 0.33 L/h/m2 and 20.6 +/- 1.6 L/m2 with an interindividual variability of 43 and 32%, respectively. The enzyme induction constant was estimated at 0.0493 +/- 0.0104 L/h2/m2. The ratio of the fraction of ifosfamide metabolised to each metabolite to the volume of distribution of that metabolite, and the elimination rate constant, of 2- and 3-dechloroethyl-ifosfamide and 4-hydroxy-ifosfamide were 0.0976 +/- 0.0556, 0.0328 +/- 0.0102 and 0.0230 +/- 0.0083 m2/L and 3.64 +/- 2.04, 0.445 +/- 0.174 and 7.67 +/- 2.87 h(-1), respectively. Interindividual variability of the first parameter was 23, 34 and 53%, respectively. Cross-validation indicated no bias and minor imprecision (12.5 +/- 5.1%) for 4-hydroxy-ifosfamide only. ... We have developed and validated a model to estimate ifosfamide and metabolite concentrations in a paediatric population by using sparse sampling.
PMID:11523727 Kerbusch T, et al; Clin Pharmacokinet 40 (8): 615-625 (2001)
... The population pharmacokinetics and pharmacodynamics of the cytostatic agent ifosfamide and its main metabolites 2- and 3-dechloroethylifosfamide and 4-hydroxyifosfamide were assessed in patients with soft tissue sarcoma. ... Twenty patients received 9 or 12 g/m2 ifosfamide administered as a 72-h continuous intravenous infusion. The population pharmacokinetic model was built in a sequential manner, starting with a covariate-free model and progressing to a covariate model with the aid of generalised additive modelling. ... The addition of the covariates weight, body surface area, albumin, serum creatinine, serum urea, alkaline phosphatase and lactate dehydrogenase improved the prediction errors of the model. Typical pretreatment (mean +/- SEM) initial clearance of ifosfamide was 3.03 +/- 0.18 l/h with a volume of distribution of 44.0 +/- 1.8 l. Autoinduction, dependent on ifosfamide levels, was characterised by an induction half-life of 11.5 +/- 1.0 h with 50% maximum induction at 33.0 +/- 3.6 microM ifosfamide. Significant pharmacokinetic-pharmacodynamic relationships (P = 0.019) were observed between the exposure to 2- and 3-dechloroethylifosfamide and orientational disorder, a neurotoxic side-effect. No pharmacokinetic-pharmacodynamic relationships between exposure to 4-hydroxyifosfamide and haematological toxicities could be observed in this population.
PMID:11699611 Kerbusch T, et al; Eur J Clin Pharmacol 57 (6-7): 467-477 (2001)
For more Absorption, Distribution and Excretion (Complete) data for IFOSFAMIDE (6 total), please visit the HSDB record page.
Primarily hepatic. Ifosfamide is metabolized through two metabolic pathways: ring oxidation ("activation") to form the active metabolite, 4-hydroxy-ifosfamide and side-chain oxidation to form the inactive metabolites, 3-dechloro-ethylifosfamide or 2-dechloroethylifosfamide with liberation of the toxic metabolite, chloroacetaldehyde. Small quantities (nmol/mL) of ifosfamide mustard and 4-hydroxyifosfamide are detectable in human plasma. Metabolism of ifosfamide is required for the generation of the biologically active species and while metabolism is extensive, it is also quite variable among patients.
Like cyclophosphamide, ifosfamide is activated in the liver by hydroxylation. However, the activation of ifosfamide proceeds more slowly, with greater production of dechlorinated metabolites & chloroacetaldehyde. These differences in metabolism likely account for the higher doses of ifosfamide required for equitoxic effects & the possible difference in antitumor spectrum of the two agents.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 1391
Like cyclophosphamide, isophosphamide requires metabolism by microsomal enzymes to act as a cytotoxic agent. It is rapidly metabolized in many species, including rodents and dogs; the urinary metabolites indicate that a series of reactions take place analogous to those in the metabolism of cyclophosphamide. Acrolein is produced during its oxidative degradation, and one product of the reaction is the ring-opened carboxy derivative. Dogs also rapidly metabolize isophosphamide, and the carboxy derivative and 4-keto isophosphamide have been identified in the urine.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V26 243 (1981)
The aim of this study was to develop a population pharmacokinetic model that could describe the pharmacokinetics of ifosfamide. 2- and 3-dechloroethylifosfamide and 4-hydroxyifosfamide, and calculate their plasma exposure and urinary excretion. A group of 14 patients with small-cell lung cancer received a 1-h intravenous infusion of 2.0 or 3.0 g/m2 ifosfamide over 1 or 2 days in combination with 175 mg/m2 paclitaxel and carboplatin at AUC 6. The concentration-time profiles of ifosfamide were described by an ifosfamide concentration-dependent development of autoinduction of ifosfamide clearance. Metabolite compartments were linked to the ifosfamide compartment enabling description of the concentration-time profiles of 2- and 3-dechloroethylifosfamide and 4-hydroxyifosfamide. The Bayesian estimates of the pharmacokinetic parameters were used to calculate the systemic exposure to ifosfamide and its metabolites for the four ifosfamide schedules. Fractionation of the dose over 2 days resulted increased metabolite formation, especially of 2-dechloroethylifosfamide, probably due to increased autoinduction. Renal recovery was only minor with 6.6% of the administered dose excreted unchanged and 9.8% as dechloroethylated metabolites. In conclusion, ifosfamide pharmacokinetics were described with an ifosfamide concentration-dependent development of autoinduction and allowed estimation of the population pharmacokinetics of the metabolites of ifosfamide. Fractionation of the dose resulted in increased exposure to 2-dechloroethylifosfamide, probably due to increased autoinduction.
PMID:11488525 Kerbusch T, et al; Cancer Chemother Pharmacol 48 (1): 53-61 (2001)
The anticancer drug ifosfamide is a prodrug requiring activation through 4-hydroxyifosfamide to ifosforamide mustard, to exert cytotoxicity. Deactivation of ifosfamide leads to 2- and 3-dechloroethylifosfamide and the release of potentially neurotoxic chloracetaldehyde. The aim of this study was to quantify and to compare the pharmacokinetics of ifosfamide, 2- and 3-dechloroethylifosfamide, 4-hydroxyifosfamide, and ifosforamide mustard in short (1-4 h), medium (24-72 h), and long infusion durations (96-240 h) of ifosfamide. An integrated population pharmacokinetic model was used to describe the autoinducible pharmacokinetics of ifosfamide and its four metabolites in 56 patients. The rate by which autoinduction of the metabolism of ifosfamide developed was found to be significantly dependent on the infusion schedule. The rate was 52% lower with long infusion durations compared with short infusion durations. This difference was, however, comparable with its interindividual variability (22%) and was, therefore, considered to be of minor clinical importance. Autoinduction caused a less than proportional increase in the area under the ifosfamide plasma concentration-time curve (AUC) and more than proportional increase in metabolite exposure with increasing ifosfamide dose. During long infusion durations dose-corrected exposures (AUC/D) were significantly decreased for ifosfamide and increased for 3-dechloroethylifosfamide compared with short infusion durations. No differences in dose-normalized exposure to ifosfamide and metabolites were observed between short and medium infusion durations. This study demonstrates that the duration of ifosfamide infusion influences the exposure to the parent and its metabolite 3-dechloroethylifosfamide. The observed dose and infusion duration dependence should be taken into account when modeling ifosfamide metabolism.
PMID:11408362 Kerbusch T, et al; Drug Metab Dispos 29 (7): 967-975 (2001)
Ifosfamide is a known human metabolite of L-trofosfamide.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
7-15 hours. The elimination half-life increase appeared to be related to the increase in ifosfamide volume of distribution with age.
The elimination half-life associated with doses of 2.5 g/sq m is 6-8 hr, whereas the elimination half-life associated with doses of 3.5-5 g/sq m is 14-16 hr.
Young, L.Y., M.A. Koda-Kimble (eds.). Applied Therapeutics. The Clinical Use of Drugs. 6th ed. Vancouver, WA., Applied Therapeutics, Inc. 1995., p. 91-22
The exact mechanism of ifosfamide has not been determined, but appears to be similar to other alkylating agents. Ifosfamide requires biotransformation in the liver by mixed-function oxidases (cytochrome P450 system) before it becomes active. After metabolic activation, active metabolites of ifosfamide alkylate or bind with many intracellular molecular structures, including nucleic acids. The cytotoxic action is primarily through the alkylation of DNA, done by attaching the N-7 position of guanine to its reactive electrophilic groups. The formation of inter and intra strand cross-links in the DNA results in cell death.
Mechanism of action: metabolites cause alkylation of DNA. /from table/
Young, L.Y., M.A. Koda-Kimble (eds.). Applied Therapeutics. The Clinical Use of Drugs. 6th ed. Vancouver, WA., Applied Therapeutics, Inc. 1995., p. 90-12
Ifosfamide, a structural analog of cyclophosphamide, belongs to the oxazaphosphorine class of antitumor alkylating agents which must be activated by the mixed function oxidase system of the liver. The 4-hydroxy oxazaphosphorines are a reactive species capable of interacting with nucleic acids & cellular materials to cause cell damage & death. The 4-hydroxy metabolite spontaneously liberates acrolein in many sites throughout the body & it is this substance that is responsible for oxazaphosphorine urotoxicity. Both ifosfamide & cyclophosphamide produce cystitis characterized by tissue edema & ulceration followed by sloughing of mucosal epithelial cells, necrosis of smooth muscle fibers & arteries, & culminating in focal hemorrhage. The selective urotoxicity of oxazaphosphorine occurs because the bladder contains a very low concn of thiol cmpds (glutathione, cysteine) which, by virtue of their nucleophilic sulfhydryl groups, are able to react & neutralize many reactive chemicals. Because the metabolic activation of ifosfamide proceeds more slowly than that of cyclophosphamide, doses of ifosfamide are 3-4 times higher than those of cyclophosphamide. This explains the higher incidence of urotoxicity associated with ifosfamide.
Young, L.Y., M.A. Koda-Kimble (eds.). Applied Therapeutics. The Clinical Use of Drugs. 6th ed. Vancouver, WA., Applied Therapeutics, Inc. 1995., p. 91-21

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
39
PharmaCompass offers a list of Ifosfamide API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Ifosfamide manufacturer or Ifosfamide supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Ifosfamide manufacturer or Ifosfamide supplier.
PharmaCompass also assists you with knowing the Ifosfamide API Price utilized in the formulation of products. Ifosfamide API Price is not always fixed or binding as the Ifosfamide Price is obtained through a variety of data sources. The Ifosfamide Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Ifosfamide manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Ifosfamide, including repackagers and relabelers. The FDA regulates Ifosfamide manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Ifosfamide API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Ifosfamide manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Ifosfamide supplier is an individual or a company that provides Ifosfamide active pharmaceutical ingredient (API) or Ifosfamide finished formulations upon request. The Ifosfamide suppliers may include Ifosfamide API manufacturers, exporters, distributors and traders.
click here to find a list of Ifosfamide suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Ifosfamide DMF (Drug Master File) is a document detailing the whole manufacturing process of Ifosfamide active pharmaceutical ingredient (API) in detail. Different forms of Ifosfamide DMFs exist exist since differing nations have different regulations, such as Ifosfamide USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Ifosfamide DMF submitted to regulatory agencies in the US is known as a USDMF. Ifosfamide USDMF includes data on Ifosfamide's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Ifosfamide USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Ifosfamide suppliers with USDMF on PharmaCompass.
A Ifosfamide CEP of the European Pharmacopoeia monograph is often referred to as a Ifosfamide Certificate of Suitability (COS). The purpose of a Ifosfamide CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Ifosfamide EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Ifosfamide to their clients by showing that a Ifosfamide CEP has been issued for it. The manufacturer submits a Ifosfamide CEP (COS) as part of the market authorization procedure, and it takes on the role of a Ifosfamide CEP holder for the record. Additionally, the data presented in the Ifosfamide CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Ifosfamide DMF.
A Ifosfamide CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Ifosfamide CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Ifosfamide suppliers with CEP (COS) on PharmaCompass.
A Ifosfamide written confirmation (Ifosfamide WC) is an official document issued by a regulatory agency to a Ifosfamide manufacturer, verifying that the manufacturing facility of a Ifosfamide active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Ifosfamide APIs or Ifosfamide finished pharmaceutical products to another nation, regulatory agencies frequently require a Ifosfamide WC (written confirmation) as part of the regulatory process.
click here to find a list of Ifosfamide suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Ifosfamide as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Ifosfamide API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Ifosfamide as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Ifosfamide and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Ifosfamide NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Ifosfamide suppliers with NDC on PharmaCompass.
Ifosfamide Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Ifosfamide GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Ifosfamide GMP manufacturer or Ifosfamide GMP API supplier for your needs.
A Ifosfamide CoA (Certificate of Analysis) is a formal document that attests to Ifosfamide's compliance with Ifosfamide specifications and serves as a tool for batch-level quality control.
Ifosfamide CoA mostly includes findings from lab analyses of a specific batch. For each Ifosfamide CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Ifosfamide may be tested according to a variety of international standards, such as European Pharmacopoeia (Ifosfamide EP), Ifosfamide JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Ifosfamide USP).
