
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
VMF
0
Europe

0
Canada

0
Australia

0
South Africa

Annual Reports
NA
Regulatory FDF Prices
NA
0
Data Compilation #PharmaFlow
0
Weekly News Recap #Phispers

1. Fanapt
2. Hp 873
3. Hp-873
4. Zomaril
1. 133454-47-4
2. Zomaril
3. Fanapt
4. Fanapta
5. Hp 873
6. Hp-873
7. Iloperidone (fanapt)
8. 4'-(3-(4-(6-fluoro-1,2-benzisoxazol-3-yl)piperidino)propoxy)-3'-methoxyacetophenone
9. 1-[4-[3-[4-(6-fluoro-1,2-benzoxazol-3-yl)piperidin-1-yl]propoxy]-3-methoxyphenyl]ethanone
10. Vpo7kj050n
11. Chembl14376
12. 1-(4-(3-(4-(6-fluorobenzo[d]isoxazol-3-yl)piperidin-1-yl)propoxy)-3-methoxyphenyl)ethanone
13. Chebi:65173
14. Ethanone, 1-(4-(3-(4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl)propoxy)-3-methoxyphenyl)-
15. Ncgc00188864-01
16. 1-[4-[3-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1- Piperidinyl]propoxy]-3-methoxyphenyl]ethanone
17. Dsstox_cid_28986
18. Dsstox_rid_83251
19. Dsstox_gsid_49060
20. 1-(4-{3-[4-(6-fluoro-benzo[d]isoxazol-3-yl)-piperidin-1-yl]-propoxy}-3-methoxy-phenyl)-ethanone
21. Fiapta
22. 1-(4-(3-(4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl)propoxy)-3-methoxyphenyl)ethanone
23. 1-(4-{3-[4-(6-fluoro-1,2-benzoxazol-3-yl)piperidin-1-yl]propoxy}-3-methoxyphenyl)ethanone
24. 1-[4-[3-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]propoxy]-3-methoxyphenyl]ethanone
25. Smr002529575
26. Fanapt (tn)
27. Cas-133454-47-4
28. Iloperidone (usan/inn)
29. Unii-vpo7kj050n
30. Iloperidona
31. Iloperidonum
32. Iloperidone [usan:inn:ban]
33. Hsdb 8207
34. Ilo-522
35. Ethanone, 1-[4-[3-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]propoxy]-3-methoxyphenyl]-
36. Iloperidone Solution
37. Iloperidone [mi]
38. Iloperidone [inn]
39. Iloperidone [usan]
40. Gtpl87
41. Iloperidone [vandf]
42. Iloperidone [mart.]
43. Iloperidone [usp-rs]
44. Iloperidone [who-dd]
45. Mls004774132
46. Mls006011943
47. Schembl115755
48. Dtxsid6049060
49. Iloperidone, >=98% (hplc)
50. Iloperidone [orange Book]
51. Bcpp000206
52. Hms3604b21
53. Hms3654j14
54. Hms3884a19
55. Hp873
56. (non-isotopelabelled)iloperidone-d6
57. Act02698
58. Bcp02042
59. Zinc1548097
60. Tox21_113610
61. Bdbm50034043
62. Mfcd00866688
63. Pdsp1_000514
64. Pdsp1_000515
65. Pdsp2_000512
66. Pdsp2_000513
67. S1483
68. Akos005146266
69. Tox21_113610_1
70. Ac-3482
71. Bcp9000774
72. Ccg-268962
73. Cs-1236
74. Db04946
75. Ncgc00188864-02
76. Ncgc00188864-10
77. 1-(4-(3-(4-(6-fluorobenzo[d]isoxazol-3-yl)piperidin-1-yl)propoxy)-3-methoxyphenyl)ethan-1-one
78. Hy-17410
79. Am20090764
80. Ft-0631148
81. Ft-0670284
82. I0926
83. Sw219279-1
84. D02666
85. Ab01274793-01
86. Ab01274793_02
87. 454i474
88. A806617
89. L001176
90. Sr-01000940095
91. J-503185
92. Q4199443
93. Sr-01000940095-2
94. Z1691545119
95. Iloperidone, United States Pharmacopeia (usp) Reference Standard
96. 1-[4-[3-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-piperidinyl]propoxy]-3-methoxyphenyl]ethanone
97. Iloperidone Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
98. 1-(4-{3-[4-(6-fluoro-1,2-benzoxazol-3-yl)piperidin-1-yl]propoxy}-3-methoxyphenyl)ethan-1-one
99. 1-(4-{3-[4-(6-fluoro-benzo[d]isoxazol-3-yl)piperidin-1-yl]-propoxy}-3-methoxy-phenyl)-ethanone
100. 1-(4-{3-[4-(6-fluoro-benzo[d]isoxazol-3-yl)piperidin-1-yl]propoxy}-3-methoxy-phenyl)-ethanone
101. 1-[4-[3-[4(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]-propoxy]-3-methoxyphenyl]-ethanone
102. 1-[4-[3-[4-(6-fluoranyl-1,2-benzoxazol-3-yl)piperidin-1-yl]propoxy]-3-methoxy-phenyl]ethanone
103. 1-[4-[3-[4-(6-fluoro-1, 2-benzisoxazol-3-yl)-1-piperidinyl]-propoxy]-3-methoxyphenyl]-ethanone
104. 1-[4-[3-[4-(6-fluoro-1,2- Benzisoxazol-3-yl)-1-piperidinyl]propoxy]-3- Methoxyphenyl]ethanone
105. 1-[4-[3-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperdinyl]propoxy]-3-methoxyphenyl]-ethanone
106. 1-[4-[3-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]- Propoxy]-3-methoxyphenyl]-ethanone
107. 1-[4-[3-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]-propoxy]-3-methoxyphenyl]-ethanone
108. 1-[4-[3-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]-propoxy]-3methoxyphenyl]-ethanone
109. 1-[4-[3-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]-propoxyl]-3-methoxyphenyl]-ethanone
110. 1-[4-[3-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]propoxy]-3-methoxy-phenyl]ethanone
111. 1-[4-[3-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]propoxy]-3-methoxyphenyl] Ethanone
112. 1-[4-[3-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]propoxy]-3-methoxyphenyl]-ethanone
113. 1-[4-[3-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]propoxy]-3methoxyphenyl]-ethanone
114. 1-[4-[3-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]propoxy]-3methoxyphenyl]ethanone
115. 1-[4-[3-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1piperidinyl]-propoxy]-3-methoxyphenyl]-ethanone
116. 1-[4-[3-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-piperidinyl]propoxy]-3-methoxyphenyl]-ethanone
117. 1-[4-[3-[4-(6-fluoro-1,2-benzisoxazol-3-yl)1-piperidinyl]-propoxy]-3-methoxyphenyl]-ethanone
118. 1-[4-[3-[4-(6-fluoro-1,2-benzisoxazol-3yl)-1-piperidinyl]propoxy]-3-methoxyphenyl] Ethanone
119. 1-[4-[3-[4-(6-fluoro-1,2-benzisoxazol-3yl)-1-piperidinyl]propoxy]-3-methoxyphenyl]-ethanone
120. 1-[4-[3-[4-(6-fluoro-1,2-benzisoxazol-3yl)-1-piperidinyl]propoxy]-3-methoxyphenyl]ethanone
121. 1-[4-[3-[4-(6-fluoro-1,2-benzoxazol-3-yl)-1-piperidinyl]propoxy]-3-methoxyphenyl]ethanone
122. 1-[4-[3-[4-(6-fluoro-1,2-benzoxazol-3-yl)piperidin-1-yl]propoxy]-3-(trideuteriomethoxy)phenyl]ethanone
| Molecular Weight | 426.5 g/mol |
|---|---|
| Molecular Formula | C24H27FN2O4 |
| XLogP3 | 4.1 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 8 |
| Exact Mass | 426.19548551 g/mol |
| Monoisotopic Mass | 426.19548551 g/mol |
| Topological Polar Surface Area | 64.8 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 586 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Fanapt |
| PubMed Health | Iloperidone (By mouth) |
| Drug Classes | Antipsychotic |
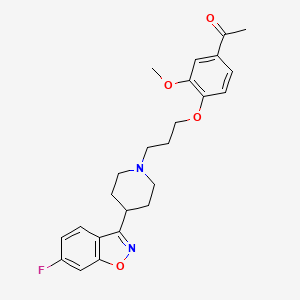
| Drug Label | FANAPT is a psychotropic agent belonging to the chemical class of piperidinyl-benzisoxazole derivatives. Its chemical name is 4-[3-[4-(6-Fluoro-1,2-benzisoxazol-3-yl)piperidino]propoxy]-3-methoxyacetophenone . Its molecular formula is C24H27FN2... |
| Active Ingredient | Iloperidone |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 4mg; 12mg; 6mg; 2mg; 10mg; 1mg; 8mg |
| Market Status | Prescription |
| Company | Novartis |
| 2 of 2 | |
|---|---|
| Drug Name | Fanapt |
| PubMed Health | Iloperidone (By mouth) |
| Drug Classes | Antipsychotic |
| Drug Label | FANAPT is a psychotropic agent belonging to the chemical class of piperidinyl-benzisoxazole derivatives. Its chemical name is 4-[3-[4-(6-Fluoro-1,2-benzisoxazol-3-yl)piperidino]propoxy]-3-methoxyacetophenone . Its molecular formula is C24H27FN2... |
| Active Ingredient | Iloperidone |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 4mg; 12mg; 6mg; 2mg; 10mg; 1mg; 8mg |
| Market Status | Prescription |
| Company | Novartis |
An atypical, negative symptom antipsychotic agent.
National Library of Medicine, SIS; ChemIDplus Lite Record for Iloperidone (133454-47-4). Available from, as of June 17, 2014: https://chem.sis.nlm.nih.gov/chemidplus/chemidlite.jsp
Fanapt tablets are indicated for the treatment of adults with schizophrenia. /Included in US product label/
NIH; DailyMed. Current Medication Information for Fanapt (Iloperidone) Tablet Fanapt (Iloperidone) Kit (Revised: April 2014). Available from, as of July 1, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=43452bf8-76e7-47a9-a5d8-41fe84d061f0
/BOXED WARNING/ WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS. Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analysis of seventeen placebo-controlled trials (modal duration 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in the drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. Fanapt is not approved for the treatment of patients with dementia-related psychosis.
NIH; DailyMed. Current Medication Information for Fanapt (Iloperidone) Tablet Fanapt (Iloperidone) Kit (Revised: April 2014). Available from, as of July 1, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=43452bf8-76e7-47a9-a5d8-41fe84d061f0
Geriatric patients with dementia-related psychosis treated with iloperidone are at an increased risk of death compared with those receiving placebo. In addition, an increased incidence of adverse cerebrovascular events (cerebrovascular accidents and transient ischemic attacks), including fatalities, has been observed in geriatric patients with dementia-related psychosis treated with certain atypical antipsychotic agents (aripiprazole, olanzapine, risperidone) in placebo-controlled studies. The manufacturer states that the safety and efficacy of iloperidone in the treatment of psychosis associated with Alzheimer's disease have not been established and that the drug is not approved for the treatment of patients with dementia-related psychosis. If a clinician decides to treat such patients with iloperidone, the manufacturer recommends that vigilance be exercised.
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 2523
An increased incidence of adverse cerebrovascular events (cerebrovascular accidents and transient ischemic attacks), including fatalities, has been observed in geriatric patients with dementia-related psychosis treated with certain atypical antipsychotic agents (aripiprazole, olanzapine, risperidone) in placebo-controlled studies. The manufacturer states that iloperidone is not approved for the treatment of patients with dementia-related psychosis.
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 2522
A potentially fatal symptom complex sometimes referred to as Neuroleptic Malignant Syndrome (NMS) has been reported in association with administration of antipsychotic drugs, including Fanapt. Clinical manifestations include hyperpyrexia, muscle rigidity, altered mental status (including catatonic signs) and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac dysrhythmia). Additional signs may include elevated creatine phosphokinase, myoglobinuria (rhabdomyolysis), and acute renal failure.
NIH; DailyMed. Current Medication Information for Fanapt (Iloperidone) Tablet Fanapt (Iloperidone) Kit (Revised: April 2014). Available from, as of July 1, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=43452bf8-76e7-47a9-a5d8-41fe84d061f0
For more Drug Warnings (Complete) data for Iloperidone (28 total), please visit the HSDB record page.
Treatment of acute schizophrenia.
FDA Label
Treatment of schizophrenia
Treatment of schizophrenia
Iloperidone shows high affinity and maximal receptor occupancy for dopamine D2 receptors in the caudate nucleus and putamen of the brains of schizophrenic patients. The improvement in cognition is attributed to iloperidone's high affinity for adrenergic receptors. Iloperidone also binds with high affinity to serotonin 5-HT2a and dopamine 3 receptors. Iloperidone binds with moderate affinity to dopamine D4, serotonin 5-HT6 and 5-HT7, and norepinephrine NE1 receptors. Furthermore, iloperidone binds with weak affinity to serotonin 5-HT1A, dopamine D1, and histamine H1 receptors.
Antipsychotic Agents
Agents that control agitated psychotic behavior, alleviate acute psychotic states, reduce psychotic symptoms, and exert a quieting effect. They are used in SCHIZOPHRENIA; senile dementia; transient psychosis following surgery; or MYOCARDIAL INFARCTION; etc. These drugs are often referred to as neuroleptics alluding to the tendency to produce neurological side effects, but not all antipsychotics are likely to produce such effects. Many of these drugs may also be effective against nausea, emesis, and pruritus. (See all compounds classified as Antipsychotic Agents.)
N05AX14
N - Nervous system
N05 - Psycholeptics
N05A - Antipsychotics
N05AX - Other antipsychotics
N05AX14 - Iloperidone
Absorption
Well absorbed from the GI tract and Cmax is reached within 2-4 hours. Steady-state concentration is achieved in 3-4 days post-administration of iloperidone. Relative bioavailability of the tablet formulation compared to oral solution is 96%. Accumulation occurs in a predictable fashion.
Route of Elimination
Renal (in which <1% of iloperidone is excreted unchanged).
Volume of Distribution
Apparent Vd = 1340-2800 L
Clearance
Apparent clearance (clearance/bioavilability) = 47-102 L/h.
Iloperidone has an apparent clearance (clearance/bioavailability) of 47 to 102 L/hr, with an apparent volume of distribution of 1340 to 2800 L. At therapeutic concentrations, the unbound fraction of iloperidone in plasma is approximately 3% and of each metabolite (P88 and P95) it is approximately 8%.
NIH; DailyMed. Current Medication Information for Fanapt (Iloperidone) Tablet Fanapt (Iloperidone) Kit (Revised: April 2014). Available from, as of July 1, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=43452bf8-76e7-47a9-a5d8-41fe84d061f0
The majority of radiolabeled iloperidone was recovered in the urine (mean 58.2% and 45.1% in extensive and poor metabolizers of CYP2D6, respectively), with feces accounting for 19.9% (extensive metabolizers) and 22.1% (poor metabolizers) of the radiolabeled dose.
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 2524
Iloperidone is well absorbed after administration of the tablet with peak plasma concentrations occurring within 2 to 4 hours; while the relative bioavailability of the tablet formulation compared to oral solution is 96%. Administration of iloperidone with a standard high-fat meal did not significantly affect the Cmax or AUC of iloperidone, P88, or P95, but delayed Tmax by 1 hour for iloperidone, 2 hours for P88 and 6 hours for P95. Fanapt can be administered without regard to meals.
NIH; DailyMed. Current Medication Information for Fanapt (Iloperidone) Tablet Fanapt (Iloperidone) Kit (Revised: April 2014). Available from, as of July 1, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=43452bf8-76e7-47a9-a5d8-41fe84d061f0
... The P88 metabolite penetrates the CNS and is thought to contribute to the drug's antipsychotic activity whereas the P95 metabolite does not readily penetrate the CNS ... .
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 2524
Iloperidone is hepatically metabolized by cytochrome enzymes which mediates O-dealkylation (CYP3A4), hydroxylation (CYP2D6), and decarboxylation/reduction processes. Metabolites formed are P89, P95, and P88. The minor metabolite is P89, whereas P95 and P88 are the major ones. The affinity of the iloperidone metabolite P88 is generally equal or less than that of the parent compound. In contrast, the metabolite P95 only shows affinity for 5-HT2A (Ki value of 3.91) and the NE1A, NE1B, NE1D, and NE2C receptors (Ki values of 4.7, 2.7, 8.8 and 4.7 nM respectively).
Iloperidone is primarily metabolized by carbonyl reduction, cytochrome P-450 (CYP) isoenzyme 2D6-mediated hydroxylation, and CYP3A4-mediated O-demethylation; the drug's two principal metabolites, P88 and P95, undergo further oxidation and/or conjugation with glucuronic acid. The P88 metabolite penetrates the CNS and is thought to contribute to the drug's antipsychotic activity whereas the P95 metabolite does not readily penetrate the CNS and primarily contributes to the adverse effect profile of the drug.
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 2524
Iloperidone is metabolized primarily by 3 biotransformation pathways: carbonyl reduction, hydroxylation (mediated by CYP2D6) and O-demethylation (mediated by CYP3A4). There are 2 predominant iloperidone metabolites, P95 and P88. The iloperidone metabolite P95 represents 47.9% of the AUC of iloperidone and its metabolites in plasma at steady-state for extensive metabolizers (EM) and 25% for poor metabolizers (PM). The active metabolite P88 accounts for 19.5% and 34.0% of total plasma exposure in EM and PM, respectively. Approximately 7% to 10% of Caucasians and 3% to 8% of black/African Americans lack the capacity to metabolize CYP2D6 substrates and are classified as poor metabolizers (PM), whereas the rest are intermediate, extensive or ultrarapid metabolizers. Co-administration of Fanapt with known strong inhibitors of CYP2D6 like fluoxetine results in a 2.3-fold increase in iloperidone plasma exposure, and therefore one-half of the Fanapt dose should be administered. Similarly, PMs of CYP2D6 have higher exposure to iloperidone compared with EMs and PMs should have their dose reduced by one-half.
NIH; DailyMed. Current Medication Information for Fanapt (Iloperidone) Tablet Fanapt (Iloperidone) Kit (Revised: April 2014). Available from, as of July 1, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=43452bf8-76e7-47a9-a5d8-41fe84d061f0
Iloperidone has known human metabolites that include 1-[4-[3-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]- propoxy]-3-hydroxyphenyl]ethanone, 1-[4-[3-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]propoxy]-3-methoxyphenyl]-2-hydroxyethanone, and 4-[3-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]propoxy]-3-methoxy-a-methylbenzene methanol.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
The observed mean elimination half-lives for iloperidone, P88 and P95 in CYP2D6 extensive metabolizers (EM) are 18, 26 and 23 hours, respectively, and in poor metabolizers (PM) are 33, 37 and 31 hours, respectively.
The mean elimination half-lives of iloperidone, P88, and P95 are 18, 26, and 23 hours, respectively, in extensive metabolizers of CYP2D6 and 33, 37, and 31 hours, respectively, in poor metabolizers of CYP2D6.
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 2524
Iloperidone is a dopamine D2 and 5-HT2A receptor antagonist and acts as a neuroleptic agent.
Iloperidone is a piperidinyl-benzisoxazole derivative structurally related to risperidone; the drug has been referred to as an atypical or second-generation antipsychotic agent. Although the exact mechanism of action of iloperidone and other antipsychotic agents in schizophrenia is unknown, it has been suggested that the efficacy of iloperidone is mediated through a combination of antagonist activity at central dopamine type 2 (D2) and serotonin type 2 (5-hydroxytryptamine (5-HT2)) receptors.
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 2524
Fanapt exhibits high (nM) affinity binding to serotonin 5-HT2A dopamine D2 and D3 receptors, and norepinephrine NEalpha1 receptors (Ki values of 5.6, 6.3, 7.1, and 0.36 nM, respectively). FANAPT has moderate affinity for dopamine D4, and serotonin 5-HT6 and 5-HT7 receptors (Ki values of 25, 43, and 22, nM respectively), and low affinity for the serotonin 5-HT1A, dopamine D1, and histamine H1 receptors (Ki values of 168, 216 and 437 nM, respectively). Fanapt has no appreciable affinity (Ki >1000 nM) for cholinergic muscarinic receptors. Fanapt functions as an antagonist at the dopamine D2, D3, serotonin 5-HT1A and norepinephrine alpha1/alpha2C receptors. The affinity of the FANAPT metabolite P88 is generally equal or less than that of the parent compound. In contrast, the metabolite P95 only shows affinity for 5-HT2A (Ki value of 3.91) and the NEalpha1A, NEalpha1B, NEalpha1D, and NEalpha2C receptors (Ki values of 4.7, 2.7, 8.8, and 4.7 nM respectively).
NIH; DailyMed. Current Medication Information for Fanapt (Iloperidone) Tablet Fanapt (Iloperidone) Kit (Revised: April 2014). Available from, as of July 1, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=43452bf8-76e7-47a9-a5d8-41fe84d061f0
Iloperidone has demonstrated an interesting monoamine receptor profile in radioligand binding studies, with nanomolar affinity for certain noradrenaline, dopamine, and serotonin receptors. In this study, the agonist/antagonist activity of iloperidone was determined in cell lines expressing recombinant human D(2A), D(3), alpha(2C), 5-HT(1A), or 5-HT(6) receptors. With the exception of 5-HT(6) receptors, these receptors are negatively coupled to cyclase. Thus, after stimulation with forskolin, the agonists dopamine (at D(2A) and D(3)), noradrenaline (at alpha(2C)), or 8-OH-DPAT (at 5-HT(1A)) induced a reduction in cAMP accumulation. Conversely, activation of the 5-HT(6) receptor by 5-HT led to an increase in cAMP accumulation. Iloperidone alone was devoid of significant agonist activity but inhibited the agonist response in all 5 cell lines in a surmountable and concentration-dependent fashion. Iloperidone was most potent at D(3) receptors (pK(B) 8.59 + or - 0.20; n = 6), followed by alpha(2C) (pK(B) 7.83 + or - 0.06; n = 15), 5-HT(1A) (pK(B) 7.69 + or - 0.18; n = 10), D(2A) (pK(B) 7.53 + or - 0.04; n = 11) and 5-HT(6) (pK(B) 7.11 + or - 0.08; n = 11) receptors.
PMID:12818723 Kalkman HO et al; Life Sci 73 (9): 1151-9 (2003)
Iloperidone ... demonstrated a potent antipsychotic profile in several in vitro and in vivo animal models. Iloperidone displaced ligand binding at D2 dopamine receptors (IC50 = 0.11 uM) and displayed a high affinity for serotonin (5-HT2) receptors (IC50 = 0.011 uM) and alpha-1 receptors (IC50 = 0.00037 uM). ...
PMID:7562515 Szewczak MR et al; J Pharmacol Exp Ther 274 (3): 1404-13 (1995)
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
44
PharmaCompass offers a list of Iloperidone API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Iloperidone manufacturer or Iloperidone supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Iloperidone manufacturer or Iloperidone supplier.
PharmaCompass also assists you with knowing the Iloperidone API Price utilized in the formulation of products. Iloperidone API Price is not always fixed or binding as the Iloperidone Price is obtained through a variety of data sources. The Iloperidone Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Iloperidone manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Iloperidone, including repackagers and relabelers. The FDA regulates Iloperidone manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Iloperidone API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Iloperidone manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Iloperidone supplier is an individual or a company that provides Iloperidone active pharmaceutical ingredient (API) or Iloperidone finished formulations upon request. The Iloperidone suppliers may include Iloperidone API manufacturers, exporters, distributors and traders.
click here to find a list of Iloperidone suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Iloperidone DMF (Drug Master File) is a document detailing the whole manufacturing process of Iloperidone active pharmaceutical ingredient (API) in detail. Different forms of Iloperidone DMFs exist exist since differing nations have different regulations, such as Iloperidone USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Iloperidone DMF submitted to regulatory agencies in the US is known as a USDMF. Iloperidone USDMF includes data on Iloperidone's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Iloperidone USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Iloperidone suppliers with USDMF on PharmaCompass.
A Iloperidone written confirmation (Iloperidone WC) is an official document issued by a regulatory agency to a Iloperidone manufacturer, verifying that the manufacturing facility of a Iloperidone active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Iloperidone APIs or Iloperidone finished pharmaceutical products to another nation, regulatory agencies frequently require a Iloperidone WC (written confirmation) as part of the regulatory process.
click here to find a list of Iloperidone suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Iloperidone as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Iloperidone API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Iloperidone as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Iloperidone and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Iloperidone NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Iloperidone suppliers with NDC on PharmaCompass.
Iloperidone Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Iloperidone GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Iloperidone GMP manufacturer or Iloperidone GMP API supplier for your needs.
A Iloperidone CoA (Certificate of Analysis) is a formal document that attests to Iloperidone's compliance with Iloperidone specifications and serves as a tool for batch-level quality control.
Iloperidone CoA mostly includes findings from lab analyses of a specific batch. For each Iloperidone CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Iloperidone may be tested according to a variety of international standards, such as European Pharmacopoeia (Iloperidone EP), Iloperidone JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Iloperidone USP).
