
Synopsis
Synopsis
0
VMF
0
Australia

0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
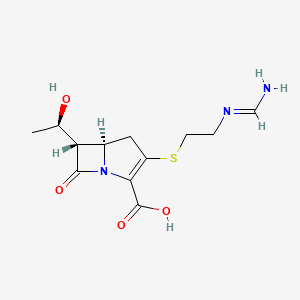

1. Anhydrous Imipenem
2. Anhydrous, Imipenem
3. Imipemide
4. Imipenem Anhydrous
5. Imipenem, Anhydrous
6. Mk 0787
7. Mk-0787
8. Mk0787
9. N Formimidoylthienamycin
10. N-formimidoylthienamycin
1. 64221-86-9
2. Imipemide
3. N-formimidoylthienamycin
4. Imipenem Anhydrous
5. Tienamycin
6. Imipenemum
7. N-formimidoyl Thienamycin
8. Imipenem Hydrate
9. Mk 0787
10. Chebi:471744
11. 74431-23-5
12. Imipenem, N-formimidoyl Thienamycin
13. (5r,6s)-3-[2-(aminomethylideneamino)ethylsulfanyl]-6-[(1r)-1-hydroxyethyl]-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic Acid
14. Imipenem (inn)
15. Imipenem [inn]
16. (5r,6s)-6-((r)-1-hydroxyethyl)-3-(2-(iminomethylamino)ethylthio)-7-oxo-1-azabicyclo(3.2.0)hept-2-ene-2-carbonsaeure
17. (5r,6s)-3-((2-(formimidoylamino)ethyl)thio)-6-((r)-1-hydroxyethyl)-7-oxo-1-azabicyclo(3.2.0)hept-2-ene-2-carboxylic Acid
18. Mk-0787
19. Chembl148
20. Q20im7he75
21. (5r,6s)-3-(2-formimidoylamino-ethylsulfanyl)-6-((r)-1-hydroxy-ethyl)-7-oxo-1-aza-bicyclo[3.2.0]hept-2-ene-2-carboxylic Acid
22. Imipenen
23. Dsstox_cid_3143
24. Dsstox_rid_76888
25. Dsstox_gsid_23143
26. Anhydrous Imipenem
27. Imipenem, Anhydrous
28. Imipenemum [latin]
29. Cas-64221-86-9
30. Sr-05000000294
31. Imipen
32. Unii-q20im7he75
33. Ncgc00016928-01
34. (5r,6s)-6-[(1r)-1-hydroxyethyl]-3-(2-methanimidamidoethylsulfanyl)-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic Acid
35. 1-azabicyclo(3.2.0)hept-2-ene-2-carboxylic Acid, 6-((1r)-1-hydroxyethyl)-3-((2-((iminomethyl)amino)ethyl)thio)-7-oxo-, (5r,6s)-
36. 1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic Acid, 6-[(1r)-1-hydroxyethyl]-3-[[2-[(iminomethyl)amino]ethyl]thio]-7-oxo-, (5r,6s)-
37. Prestwick_844
38. Einecs 264-734-5
39. Mk-787
40. Mk0787
41. Imipenem [mi]
42. Imipenem Anhydrate
43. Prestwick0_000519
44. Prestwick1_000519
45. Prestwick2_000519
46. Prestwick3_000519
47. Epitope Id:120384
48. Imipenem [who-dd]
49. Bspbio_000477
50. Bidd:gt0686
51. Spbio_002398
52. Bpbio1_000525
53. Schembl1649260
54. Schembl8781920
55. Dtxsid2023143
56. Gtpl10821
57. Hy-b1369a
58. Primaxin (imipenem + Cilastatin)
59. Hms1569h19
60. Hms2090a15
61. Hms2096h19
62. Hms3260h20
63. Hms3713h19
64. Pharmakon1600-01506001
65. Bcp13012
66. Zinc4097225
67. Tox21_110689
68. Tox21_500279
69. Bdbm50049708
70. Bdbm50213266
71. Nsc717864
72. Nsc759901
73. Akos016010844
74. Tox21_110689_1
75. Ccg-220519
76. Ccg-221583
77. Db01598
78. Lp00279
79. Nsc-717864
80. Sdccgsbi-0633697.p001
81. Ncgc00167958-01
82. Ncgc00167958-02
83. Ncgc00167958-03
84. Ncgc00167958-05
85. Ncgc00167958-09
86. Ncgc00260964-01
87. (5r,6s)-6-[(1r)-1-hydroxyethyl]-3-({2-[(iminomethyl)amino]ethyl}thio)-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic Acid
88. 1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic Acid,6-[(1r)-1-hydroxyethyl]-3-[[2-[(iminomethyl)amino]ethyl]thio]-7-oxo-,(5r,6s)-
89. As-75130
90. Cs-0077844
91. C06665
92. D04515
93. D96091
94. Ab01563339_01
95. Recarbrio (imipenem + Cilastatin + Relebactam).
96. 847i667
97. Q425152
98. Sr-05000000294-2
99. Sr-05000000294-5
100. Thienamycin P-nitrobenzylester Hydrochloride(n-methylpyrrolidinonesolvate)
101. (5r,6s)-3-((2-formimidamidoethyl)thio)-6-((r)-1-hydroxyethyl)-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic Acid
102. (5r,6s)-3-({2-[(e)-(aminomethylidene)amino]ethyl}sulfanyl)-6-[(1r)-1-hydroxyethyl]-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic Acid
103. (5r,6s)-3-(2-formimidamidoethylthio)-6-((r)-1-hydroxyethyl)-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic Acid
104. (5r,6s)-6-[(1r)-1-hydroxyethyl]-3-[(2-methanimidamidoethyl)sulfanyl]-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic Acid
105. [5r-[5.alpha.,6.alpha.(r*)]]-6-(1-hydroxyethyl)-3-[[2- [(iminomethyl)amino]ethyl]thio]-7-oxo-1-azabicyclo[3.2.0]hept-2- Ene-2-carboxylic Acid Monohydrate
106. 1-azabicyclo(3.2.0)hept-2-ene-2-carboxylic Acid, 6-(1-hydroxyethyl)-3-((2-((iminomethyl)amino)ethyl)thio)-7-oxo-, (5r-(5-alpha,6-alpha(r*)))-
107. 1-azabicyclo(3.2.0)hept-2-ene-2-carboxylic Acid, 6-(1-hydroxyethyl)-3-((2-((iminomethyl)amino)ethyl)thio)-7-oxo-, (5r-(5.alpha.,6.alpha.(r*)))-
108. 103730-39-8
109. 3-[(2-aminoethyl)thio]-6-[(1r)-1-hydroxyethyl]-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic Acid (4-nitrophenyl)methylester Monohydrochloride Compd. With 1-methyl-2-pyrrolidinone (1:1)
| Molecular Weight | 299.35 g/mol |
|---|---|
| Molecular Formula | C12H17N3O4S |
| XLogP3 | -0.7 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 6 |
| Exact Mass | 299.09397721 g/mol |
| Monoisotopic Mass | 299.09397721 g/mol |
| Topological Polar Surface Area | 142 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 491 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Primaxin |
| PubMed Health | Imipenem/Cilastatin (Injection) |
| Drug Classes | Antibiotic |
| Active Ingredient | imipenem; Cilastatin sodium |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 500mg/vial; 250mg/vial; eq 500mg base/vial; eq 250mg base/vial |
| Market Status | Prescription |
| Company | Merck |
| 2 of 2 | |
|---|---|
| Drug Name | Primaxin |
| PubMed Health | Imipenem/Cilastatin (Injection) |
| Drug Classes | Antibiotic |
| Active Ingredient | imipenem; Cilastatin sodium |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 500mg/vial; 250mg/vial; eq 500mg base/vial; eq 250mg base/vial |
| Market Status | Prescription |
| Company | Merck |
Imipenem is indicated, in combination with [cilastatin] with or without [relebactam], for the treatment of bacterial infections including respiratory, skin, bone, gynecologic, urinary tract, and intra-abdominal as well as septicemia and endocarditis.
FDA Label
Imipenem is a beta-lactam antibiotic belongings to the subgroup of carbapenems. Imipenem is active against aerobic and anaerobic Gram positive as well as Gram negative bacteria including Pseudomonas aeruginosa and the Enterococcus. It exerts a bactericidal effects by disrupting cell wall synthesis.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J01DH51
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
Absorption
Imipenem is not effectively absorbed from the gastrointestinal tract and therefore must be administered parenterally. The bioavailability of the IM injection is 89%.
Route of Elimination
Approximately 70% of imipenem is excreted in the urine as the parent drug.
Volume of Distribution
The reported volume of distribution for imipenem ranges from 0.23-0.31 L/kg.
Clearance
The total clearance of imipenem is 0.2 L/h/kg. When used alone, the renal clearance is 0.05 L/h/kg. In combination with cilastatin the renal clearance of imipenem is 0.15 L/h/kg, likely due to the increased concentration of the parent drug.
Imipenem is metabolized by renal dehydropeptidase.
When given via IV injection imipenem has a half-life of 1 h. The apparent half-life of the IM injection ranges from 1.3-5.1 h, likely due to slower absorption form the injection site.
Imipenem acts as an antimicrobial through the inhibition of cell wall synthesis of various gram-positive and gram-negative bacteria. This inhibition of cell wall synthesis in gram-negative bateria is attained by binding to penicillin-binding proteins (PBPs). In E. coli and selected strains of P. aeruginosa, imipenem has shown to have the highest affinity to PBP-2, PBP-1a, and PBP-1b. This inhibition of PBPs prevents the bacterial cell from adding to the peptidoglycan polymer which forms the bacterial cell wall eventually leading to cell death.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
77
PharmaCompass offers a list of Imipenem API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Imipenem manufacturer or Imipenem supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Imipenem manufacturer or Imipenem supplier.
PharmaCompass also assists you with knowing the Imipenem API Price utilized in the formulation of products. Imipenem API Price is not always fixed or binding as the Imipenem Price is obtained through a variety of data sources. The Imipenem Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Imipenem manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Imipenem, including repackagers and relabelers. The FDA regulates Imipenem manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Imipenem API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Imipenem manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Imipenem supplier is an individual or a company that provides Imipenem active pharmaceutical ingredient (API) or Imipenem finished formulations upon request. The Imipenem suppliers may include Imipenem API manufacturers, exporters, distributors and traders.
click here to find a list of Imipenem suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Imipenem DMF (Drug Master File) is a document detailing the whole manufacturing process of Imipenem active pharmaceutical ingredient (API) in detail. Different forms of Imipenem DMFs exist exist since differing nations have different regulations, such as Imipenem USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Imipenem DMF submitted to regulatory agencies in the US is known as a USDMF. Imipenem USDMF includes data on Imipenem's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Imipenem USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Imipenem suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Imipenem Drug Master File in Japan (Imipenem JDMF) empowers Imipenem API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Imipenem JDMF during the approval evaluation for pharmaceutical products. At the time of Imipenem JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Imipenem suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Imipenem Drug Master File in Korea (Imipenem KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Imipenem. The MFDS reviews the Imipenem KDMF as part of the drug registration process and uses the information provided in the Imipenem KDMF to evaluate the safety and efficacy of the drug.
After submitting a Imipenem KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Imipenem API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Imipenem suppliers with KDMF on PharmaCompass.
A Imipenem CEP of the European Pharmacopoeia monograph is often referred to as a Imipenem Certificate of Suitability (COS). The purpose of a Imipenem CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Imipenem EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Imipenem to their clients by showing that a Imipenem CEP has been issued for it. The manufacturer submits a Imipenem CEP (COS) as part of the market authorization procedure, and it takes on the role of a Imipenem CEP holder for the record. Additionally, the data presented in the Imipenem CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Imipenem DMF.
A Imipenem CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Imipenem CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Imipenem suppliers with CEP (COS) on PharmaCompass.
A Imipenem written confirmation (Imipenem WC) is an official document issued by a regulatory agency to a Imipenem manufacturer, verifying that the manufacturing facility of a Imipenem active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Imipenem APIs or Imipenem finished pharmaceutical products to another nation, regulatory agencies frequently require a Imipenem WC (written confirmation) as part of the regulatory process.
click here to find a list of Imipenem suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Imipenem as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Imipenem API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Imipenem as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Imipenem and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Imipenem NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Imipenem suppliers with NDC on PharmaCompass.
Imipenem Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Imipenem GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Imipenem GMP manufacturer or Imipenem GMP API supplier for your needs.
A Imipenem CoA (Certificate of Analysis) is a formal document that attests to Imipenem's compliance with Imipenem specifications and serves as a tool for batch-level quality control.
Imipenem CoA mostly includes findings from lab analyses of a specific batch. For each Imipenem CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Imipenem may be tested according to a variety of international standards, such as European Pharmacopoeia (Imipenem EP), Imipenem JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Imipenem USP).
