

Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
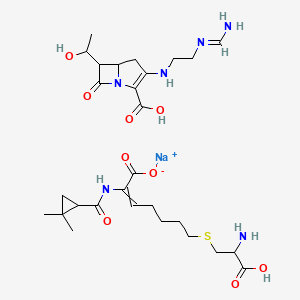

| Molecular Weight | 662.7 g/mol |
|---|---|
| Molecular Formula | C28H43N6NaO9S |
| Hydrogen Bond Donor Count | 7 |
| Hydrogen Bond Acceptor Count | 13 |
| Rotatable Bond Count | 17 |
| Exact Mass | 662.27099243 g/mol |
| Monoisotopic Mass | 662.27099243 g/mol |
| Topological Polar Surface Area | 286 Ų |
| Heavy Atom Count | 45 |
| Formal Charge | 0 |
| Complexity | 1010 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 5 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 1 |
| Covalently Bonded Unit Count | 3 |
 Click Us!
Click Us! 
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 21004
Submission : 2007-10-26
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 21707
Submission : 2008-07-25
Status : Inactive
Type : II




 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 21707
Submission : 2008-07-25
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 21004
Submission : 2007-10-26
Status : Active
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
Imipenem And Cilastatin Sodium
About the Company : We focus on development of carbapenem APIs. In hospital, these drugs are used for complicated infections. As a last-line antibiotic, the consumption is increased every year attribu...

Sterile Imipenem/ Cilastatin Sodium
About the Company : Since its foundation in 1959, JEIL Pharm established. its own central research laboratory for the formulation and synthesis of drugs, followed by its successive construction of KGM...

Imipenem And Cilastatin Sodium
About the Company : The company was formerly known as "Choongwae Pharma Corporation" and changed its name to JW Pharmaceutical on April 21, 2011. JW Pharmaceutical was founded in 1945 and is based in ...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Market Place

Reply
02 May 2022
Reply
27 Oct 2018
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
