
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
VMF
0
Canada

0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. 4,4'-methylenebis(3-hydroxy-2-naphthoic Acid)-3-(10,11-dihydro-5h-dibenzo(b,f)azepin-5-yl)-n,n-dimethyl-1-propanamine (1:2)
2. Imidobenzyle
3. Imipramine Hydrochloride
4. Imipramine Monohydrochloride
5. Imipramine Pamoate
6. Imizin
7. Janimine
8. Melipramine
9. Norchlorimipramine
10. Pryleugan
11. Tofranil
1. Melipramine
2. 50-49-7
3. Antideprin
4. Berkomine
5. Imidobenzyle
6. Dimipressin
7. Melipramin
8. Tofranil
9. Intalpram
10. Nelipramin
11. Dynaprin
12. Timolet
13. Irmin
14. Dpid
15. Dyna-zina
16. Impramine
17. Promiben
18. Censtim
19. Censtin
20. Imiprin
21. Iramil
22. Janimine
23. Imipramina
24. 3-(10,11-dihydro-5h-dibenzo[b,f]azepin-5-yl)-n,n-dimethylpropan-1-amine
25. Declomipramine
26. Eupramin
27. Imipramin
28. Imipraminum
29. Psychoforin
30. Imavate
31. Surplix
32. Imizin
33. Tofranil, Base
34. Imizinum
35. N-(gamma-dimethylaminopropyl)iminodibenzyl
36. N-(3-dimethylaminopropyl)-o-iminodibenzyl
37. 2,2'-(3-dimethylaminopropylimino)bibenzyl
38. 2,2'-(3-dimethylaminopropylimino)dibenzyl
39. Tofranil (free Base)
40. Sk-pramine
41. Cristalia
42. Sermonil
43. Imipramine (inn)
44. Nsc 169866
45. 5-(3-(dimethylamino)propyl)-10,11-dihydro-5h-dibenz(b,f)azepine
46. 3-(5,6-dihydrobenzo[b][1]benzazepin-11-yl)-n,n-dimethylpropan-1-amine
47. Chembl11
48. 1-(3-dimethylaminopropyl)-4,5-dihydro-2,3,6,7-dibenzazepine
49. Nsc-169866
50. 10,11-dihydro-n,n-dimethyl-5h-dibenz[b,f]azepine-5-propanamine
51. 5,6-dihydro-n-(3-(dimethylamino)propyl)-11h-dibenz(b,e)azepine
52. 5-(3-dimethylaminopropyl)-10,11-dihydro-5h-dibenzo(b,f)azepine
53. 5h-dibenz(b,f)azepine-5-propanamine, 10,11-dihydro-n,n-dimethyl-
54. Ogg85sx4e4
55. N-(.gamma.-dimethylaminopropyl)iminodibenzyl
56. Chebi:47499
57. 5,e)azepine
58. 5,e]azepine
59. 5h-dibenz(b,f)azepine, 10,11-dihydro-5-(3-(dimethylamino)propyl)-
60. Org-2463
61. Imidol
62. Ncgc00015563-07
63. Imipramina [italian]
64. Imipramine [inn]
65. Tofranil (tn)
66. 10,11-dihydro-5-(3-(dimethylamino)propyl)-5h-dibenz[b,f]azepine
67. 3-(5h-dibenzo[b,f]azepin-5-yl)-n,n-dimethylpropan-1-amine
68. 5h-dibenz[b,f]azepine-5-propanamine, 10,11-dihydro-n,n-dimethyl-
69. 5h-dibenz(b,f)azepine, 5-(3-(dimethylamino)propyl)-10,11-dihydro-
70. Imipramine [inn:ban]
71. Dsstox_cid_23881
72. Dsstox_rid_80080
73. Dsstox_gsid_43881
74. Imipraminum [inn-latin]
75. Imipramina [inn-spanish]
76. 3-(10,11-dihydro-5h-dibenz[b,f]azepin-5-yl)propyldimethylamine
77. 5-[3-(dimethylamino)propyl]-10,11-dihydro-5h-dibenz[b,f]azepine
78. Cas-50-49-7
79. 3-(10,11-dihydro-5h-dibenzo[b,f]azepin-5-yl)-n,n-dimethylpropan-1-amine;3-(10,11-dihydro-5h-dibenzo[b,f]azepin-5-yl)-n,n-dimethylpropan-1-amine
80. N,n-dimethyl-10,11-dihydro-5h-dibenzo[b,f]azepine-5-propanamine-2,8-d2
81. Ccris 9173
82. 2241983-10-6
83. Hsdb 3100
84. 3-(5,6-dihydrobenzo[b][1]benzazepin-11-yl)-n,n-dimethyl-propan-1-amine
85. Janimine (hydrochloride)
86. Tofranil (hydrochloride)
87. Cas-113-52-0
88. Einecs 200-042-1
89. Unii-ogg85sx4e4
90. Brn 0256892
91. 5h-dibenz(b, 5-(3-(dimethylamino)propyl)-10,11-dihydro-
92. 5h-dibenz[b, 5-[3-(dimethylamino)propyl]-10,11-dihydro-
93. Tofranil Base
94. 5h-dibenz[b,f]azepine, 5-[3-(dimethylamino)propyl]-10,11-dihydro-
95. Ixx
96. Imizin (salt/mix)
97. Imavate (salt/mix)
98. Imizine (salt/mix)
99. Surplix (salt/mix)
100. Eupramin (salt/mix)
101. Imizinum (salt/mix)
102. Tofranil (salt/mix)
103. Spectrum_000915
104. Imipramine [mi]
105. Psychoforin (salt/mix)
106. Prestwick0_000072
107. Prestwick1_000072
108. Prestwick2_000072
109. Prestwick3_000072
110. Sk-pramine (salt/mix)
111. Spectrum2_000990
112. Spectrum3_000466
113. Spectrum4_000016
114. Spectrum5_000864
115. Imipramine [hsdb]
116. Lopac-i-7379
117. Imipramine [vandf]
118. 3-(10,11-dihydro-5h-dibenzo[b,f]azepin-5-yl)-n,n-dimethyl-1-propanamine
119. Imipramine [mart.]
120. Imipramine [who-dd]
121. Lopac0_000702
122. Oprea1_200908
123. Schembl34282
124. Bspbio_000283
125. Bspbio_002172
126. Gtpl357
127. Kbiogr_000391
128. Kbioss_001395
129. Bidd:gt0116
130. Divk1c_000559
131. Spbio_001059
132. Spbio_002204
133. G-22355 (salt/mix)
134. Bpbio1_000313
135. Clomipramine Hcl Ep Impurity B
136. Dtxsid1043881
137. Hy-b1490a
138. Kbio1_000559
139. Kbio2_001395
140. Kbio2_003963
141. Kbio2_006531
142. Kbio3_001392
143. Zinc20245
144. Ninds_000559
145. Hms2089g08
146. Tox21_110174
147. Bdbm50010859
148. Ccg-36485
149. Mfcd31699979
150. Nsc169866
151. Stl416211
152. Akos016010320
153. Tox21_110174_1
154. Db00458
155. Sdccgsbi-0050680.p005
156. Idi1_000559
157. Mrf-0000592
158. Ncgc00015563-01
159. Ncgc00015563-02
160. Ncgc00015563-03
161. Ncgc00015563-04
162. Ncgc00015563-05
163. Ncgc00015563-06
164. Ncgc00015563-08
165. Ncgc00015563-09
166. Ncgc00015563-10
167. Ncgc00015563-11
168. Ncgc00015563-13
169. Ncgc00015563-25
170. Ncgc00024253-03
171. Ncgc00024253-04
172. 5-(3-dimethylaminopropyl)-10,f)azepine
173. Sy246340
174. Trimipramine Maleate Impurity, Imipramine-
175. Sbi-0050680.p004
176. Wln: T C676 Bn&t&j B3n1&1
177. 5-(3-(dimethylamino)propyl)-10,f)azepine
178. Ab00053486
179. Cs-0013621
180. Ft-0670319
181. Ft-0697093
182. 5h-dibenz[b, 10,11-dihydro-n,n-dimethyl-
183. C07049
184. D08070
185. Q58396
186. Ab00053486-15
187. Ab00053486_16
188. Ab00053486_17
189. 1-(3-dimethylaminopropyl)-4,3,6,7-dibenzazepine
190. L000739
191. W-109253
192. Brd-k38436528-003-05-5
193. Brd-k38436528-003-15-4
194. Trimipramine Maleate Impurity D [ep Impurity]
195. 5h-dibenz(b, 10,11-dihydro-5-(3-(dimethylamino)propyl)-
196. Clomipramine Hydrochloride Impurity B [ep Impurity]
197. Trimipramine Maleate Impurity, Imipramine- [usp Impurity]
198. 3-(10,11-dihydro-5h-dibenzo[b,f]azepin-5-yl)-n,n-dimethyl-1-propanamine #
199. 5h-dibenz(b,5-[3-(dimethylamino)propyl]-10,11-dihydro-mixed With Ethyl Alcohol
200. Clomipramine Ep Impurity B; 10,11-dihydro-n,n-dimethyl-5h-dibenz[b,f]azepine-5-propanamine
201. (3-{2-azatricyclo[9.4.0.0^{3,8}]pentadeca-1(11),3(8),4,6,12,14-hexaen-2-yl}propyl)dimethylamine
202. 5-(3-(dimethylamino)propyl)-10,11-dihydro-5h-dibenz[b,f]azepine;10,11-dehydroimipramine;depramine
| Molecular Weight | 280.4 g/mol |
|---|---|
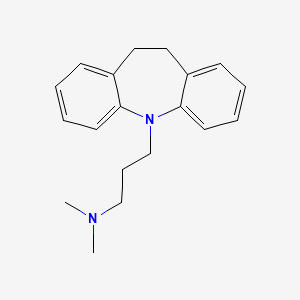
| Molecular Formula | C19H24N2 |
| XLogP3 | 4.8 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 4 |
| Exact Mass | 280.193948774 g/mol |
| Monoisotopic Mass | 280.193948774 g/mol |
| Topological Polar Surface Area | 6.5 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 291 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Tofranil |
| PubMed Health | Imipramine (By mouth) |
| Drug Classes | Antidepressant, Urinary Enuresis Agent |
| Drug Label | Tofranil is supplied in tablet form for oral administration.Tofranil, imipramine hydrochloride USP, the original tricyclic antidepressant, is a member of the dibenzazepine group of compounds. It is designated 5-3-(dimethylamino)propyl-10,11-dihydr... |
| Active Ingredient | Imipramine hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 25mg; 50mg; 10mg |
| Market Status | Prescription |
| Company | Mallinckrodt |
| 2 of 4 | |
|---|---|
| Drug Name | Tofranil-pm |
| Active Ingredient | Imipramine pamoate |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | eq 75mg hcl; eq 125mg hcl; eq 100mg hcl; eq 150mg hcl |
| Market Status | Prescription |
| Company | Mallinckrodt |
| 3 of 4 | |
|---|---|
| Drug Name | Tofranil |
| PubMed Health | Imipramine (By mouth) |
| Drug Classes | Antidepressant, Urinary Enuresis Agent |
| Drug Label | Tofranil is supplied in tablet form for oral administration.Tofranil, imipramine hydrochloride USP, the original tricyclic antidepressant, is a member of the dibenzazepine group of compounds. It is designated 5-3-(dimethylamino)propyl-10,11-dihydr... |
| Active Ingredient | Imipramine hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 25mg; 50mg; 10mg |
| Market Status | Prescription |
| Company | Mallinckrodt |
| 4 of 4 | |
|---|---|
| Drug Name | Tofranil-pm |
| Active Ingredient | Imipramine pamoate |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | eq 75mg hcl; eq 125mg hcl; eq 100mg hcl; eq 150mg hcl |
| Market Status | Prescription |
| Company | Mallinckrodt |
Adrenergic Uptake Inhibitors; Antidepressive Agents, Tricyclic
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
EFFECTIVE IN DEPRESSIVE SYNDROMES, PARTICULARLY THOSE ASSOC WITH MANIC-DEPRESSIVE & INVOLUTIONAL PSYCHOSES... /HYDROCHLORIDE/
Osol, A. (ed.). Remington's Pharmaceutical Sciences. 16th ed. Easton, Pennsylvania: Mack Publishing Co., 1980., p. 1040
2-HYDROXYIMIPRAMINE-HCL INHIBITED UPTAKE OF NOREPINEPHRINE & SEROTONIN BY RAT CEREBROCORTICAL SYNAPTOSOMES TO SAME EXTENT AS PARENT DRUGS.
POTTER WZ ET AL; BIOL PSYCHIATRY 14(4) 601 (1979)
METHODS OF TREATMENT OF ENURESIS IN CHILD ARE PRESENTED INCL IMIPRAMINE.
BINDELGLAS RM ET AL; J FAM PRACT 2 (OCT) 375 (1975)
For more Therapeutic Uses (Complete) data for IMIPRAMINE (8 total), please visit the HSDB record page.
BECAUSE OF POSSIBLE CONGENITAL MALFORMATIONS ASSOC WITH USE OF THIS DRUG.../IT/ SHOULD NOT BE USED DURING FIRST TRIMESTER OF PREGNANCY. /IMIPRAMINE HYDROCHLORIDE/
Osol, A. (ed.). Remington's Pharmaceutical Sciences. 16th ed. Easton, Pennsylvania: Mack Publishing Co., 1980., p. 1040
TRICYCLIC COMPD ARE CONTRAINDICATED IN PT WITH CONGESTIVE HEART FAILURE, ANGINA PECTORIS, & PAROXYSMAL TACHYCARDIA; ALSO, THEY SHOULD BE USED WITH CAUTION IN PATIENTS WITH URINARY RETENTION, GLAUCOMA, DIABETES, IMPAIRED LIVER FUNCTION, ASTHMA, AND A HISTORY OF CONVULSIVE SEIZURES. /TRICYCLIC ANTIDEPRESSANTS/
Osol, A. (ed.). Remington's Pharmaceutical Sciences. 16th ed. Easton, Pennsylvania: Mack Publishing Co., 1980., p. 1038
OCCASIONAL PT WILL SHOW PHYSICAL DEPENDENCE ON TRICYCLIC ANTIDEPRESSANTS, WITH MALAISE, CHILLS, CORYZA, & MUSCLE ACHES FOLLOWING ABRUPT DISCONTINUATION OF HIGH DOSES OF IMIPRAMINE.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 441
ALTHOUGH MOST FATAL CASES...HAVE OCCURRED AFTER INGESTION OF MORE THAN 1.5 G ... DEATH HAS BEEN REPORTED AFTER AS LITTLE AS 500 TO 750 MG AND RECOVERY HAS BEEN REPORTED AFTER INGESTION OF 5.4 G. /IMIPRAMINE HYDROCHLORIDE/
American Medical Association, AMA Department of Drugs. AMA Drug Evaluations. 5th ed. Chicago: American Medical Association, 1983., p. 256
For more Drug Warnings (Complete) data for IMIPRAMINE (33 total), please visit the HSDB record page.
4. 4= VERY TOXIC: PROBABLE ORAL LETHAL DOSE (HUMAN) 50-500 MG/KG; BETWEEN 1 TEASPOON AND 1 OZ FOR 70 KG PERSON (150 LB).
Gosselin, R.E., H.C. Hodge, R.P. Smith, and M.N. Gleason. Clinical Toxicology of Commercial Products. 4th ed. Baltimore: Williams and Wilkins, 1976., p. II-229
For the relief of symptoms of depression and as temporary adjunctive therapy in reducing enuresis in children aged 6 years and older. May also be used off-label to manage panic disorders with or without agoraphobia, as a second line agent for ADHD in children and adolescents, to manage bulimia nervosa, for short-term management of acute depressive episodes in bipolar disorder and schizophrenia, for the treatment of acute stress disorder and posttraumatic stress disorder, and for symptomatic treatment of postherpetic neuralgia and painful diabetic neuropathy.
FDA Label
Imipramine is a tricyclic antidepressant with general pharmacological properties similar to those of structurally related tricyclic antidepressant drugs such as amitriptyline and doxepin. While it acts to block both, imipramine displays a much higher affinity for the serotonin reuptake transporter than for the norepinephrine reuptake transporter. Imipramine produces effects similar to other monoamine targeting antidepressants, increasing serotonin- and norepinephrine-based neurotransmission. This modulation of neurotransmission produces a complex range of changes in brain structure and function along with an improvement in depressive symptoms. The changes include increases in hippocampal neurogenesis and reduced downregulation of this neurogenesis in response to stress. These implicate brain derived neurotrophic factor signalling as a necessary contributor to antidepressant effect although the link to the direct increase in monoamine neurotransmission is unclear. Serotonin reuptake targeting agents may also produce a down-regulation in -adrenergic receptors in the brain.
Adrenergic Uptake Inhibitors
Drugs that block the transport of adrenergic transmitters into axon terminals or into storage vesicles within terminals. The tricyclic antidepressants (ANTIDEPRESSIVE AGENTS, TRICYCLIC) and amphetamines are among the therapeutically important drugs that may act via inhibition of adrenergic transport. Many of these drugs also block transport of serotonin. (See all compounds classified as Adrenergic Uptake Inhibitors.)
Antidepressive Agents, Tricyclic
Substances that contain a fused three-ring moiety and are used in the treatment of depression. These drugs block the uptake of norepinephrine and serotonin into axon terminals and may block some subtypes of serotonin, adrenergic, and histamine receptors. However, the mechanism of their antidepressant effects is not clear because the therapeutic effects usually take weeks to develop and may reflect compensatory changes in the central nervous system. (See all compounds classified as Antidepressive Agents, Tricyclic.)
N - Nervous system
N06 - Psychoanaleptics
N06A - Antidepressants
N06AA - Non-selective monoamine reuptake inhibitors
N06AA02 - Imipramine
Absorption
Rapidly and well absorbed (>95%) after oral administration. The primary site of absorption is the small intestine as the basic amine groups are ionized in the acidic environment of the stomach, preventing movement across tissues. Bioavailability ranges from 29-77% due to high inter-individual variability. Peak plasma concentration is usually attained 2-6 hours following oral administration. Absorption is unaffected by food.
Route of Elimination
Imipramine is primarily excreted in the urine with less than 5% present as the parent compound
Volume of Distribution
Imipramine has a high apparent volume of distribution of 10-20 L/kg. The drug is known to accumulate in the brain at concentrations 30-40 times that in systemic circulation.
Clearance
Imipramine has a mean clearance of 1 L/h/kg. Its active metabolite, desipramine has a mean clearance of 1.8 L/h/kg.
TRICYCLIC ANTIDEPRESSANTS ARE FAIRLY WELL ABSORBED AFTER ORAL ADMINISTRATION. ... ONCE ABSORBED /IT/ IS WIDELY DISTRIBUTED. ... ARE STRONGLY BOUND TO PLASMA PROTEIN AND TO THE CONSTITUENTS OF TISSUES./TRICYCLIC ANTIDEPRESSANTS
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 440
EXCRETION...IS RAPID... APPROX 40% OF DOSE OF RADIOACTIVE IMIPRAMINE APPEARS IN URINE IN 24 HR & TOTAL OF 70% DURING FIRST 72 HR. REMAINDER APPEARS IN FECES. SMALL PORTION...RECOVERED AS UNCHANGED DRUG OR AS ACTIVE DESMETHYL DERIV. LARGER PORTION...EXCRETED AS N-OXIDE OR AS NONCONJUGATED OR CONJUGATED 2-OH DERIV.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 178
IN ANIMALS, PLACENTAL TRANSFER HAS BEEN OBSERVED WITH IMIPRAMINE & ITS DESMETHYL DERIVATIVE.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 2: A Review of the Literature Published Between 1970 and 1971. London: The Chemical Society, 1972., p. 433
DISTRIBUTION OF IV ADMIN.../(14)C, IMIPRAMINE/ IN MICE...STUDIED USING WHOLE-BODY AUTORADIOGRAPHY. 5 MIN AFTER DOSING, HIGH UPTAKE OF (14)C OCCURRED IN BRAIN, MYOCARDIUM, LUNGS, ADRENALS & KIDNEYS, BUT (14)C IN BLOOD WAS LOW. IN 1 HR...(14)C /LEVELS/...HIGH IN SALIVARY GLANDS, INTESTINES, LIVER, GALL BLADDER, & URINARY BLADDER & 3 HR LATER...LARGELY CONFINED TO ORGANS CONCERNED WITH EXCRETION OF IMIPRAMINE...INTESTINES, LIVER, & KIDNEYS.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 1: A Review of the Literature Published Between 1960 and 1969. London: The Chemical Society, 1970., p. 57
For more Absorption, Distribution and Excretion (Complete) data for IMIPRAMINE (8 total), please visit the HSDB record page.
Imipramine is nearly exclusively metabolized by the liver. Imipramine is converted to desipramine by CYP1A2, CYP3A4, CYP2C19. Both imipramine and desipramine are hydroxylated by CYP2D6. Desipramine is an active metabolite. Minor metabolic pathways include dealkylation to form an imidodibenzyl product as well as demethylation of desipramine to didemethylimipramine and subsequent hydroxylation. Less than 5% of orally administered imipramine is excreted unchanged.
...STUDY OF METABOLISM OF IMIPRAMINE & ITS METABOLITES BY RAT LIVER MICROSOMES...REVEALED OPERATION OF 16 METABOLIC PATHWAYS, INCL N-DEMETHYLATION, AROMATIC HYDROXYLATIONS, SIDE-CHAIN DEALKYLATIONS, N-OXIDATION, N-OXIDE REDUCTION, & CONJUGATION REACTIONS.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 1: A Review of the Literature Published Between 1960 and 1969. London: The Chemical Society, 1970., p. 317
IMIPRAMINE N-OXIDE & IMINODIBENZYL...IDENTIFIED AS ADDITIONAL URINARY METABOLITES IN MAN.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 1: A Review of the Literature Published Between 1960 and 1969. London: The Chemical Society, 1970., p. 183
...METABOLIZED IN HUMANS BY N-DEMETHYLATION & BY HYDROXYLATION IN ONE OF THE AROMATIC RINGS OR IN ETHYLENE BRIDGE TO GIVE DESMONOMETHYLIMIPRAMINE (DMI) & DESDIMETHYLIMIPRAMINE (DDMI) & THE 2-HYDROXY & 10-HYDROXY DERIVATIVES OF IMIPRAMINE, DMI & DDMI, TOGETHER WITH THEIR GLUCURONIDE CONJUGATES.
Parke, D. V. The Biochemistry of Foreign Compounds. Oxford: Pergamon Press, 1968., p. 192
IMIPRAMINE (HALF-LIFE, 16 HOURS) IS BIOTRANSFORMED TO THE ACTIVE METABOLITE, DESIPRAMINE (HALF-LIFE, 18 HOURS).
American Medical Association, AMA Department of Drugs. AMA Drug Evaluations. 5th ed. Chicago: American Medical Association, 1983., p. 779
Imipramine has known human metabolites that include 2-Hydroxyimipramine, Desipramine, and Imipramine N-glucuronide.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Imipramine has a mean half life of 12 h. Its active metabolite, desipramine has a mean half life of 22.5 h.
IMIPRAMINE (HALF-LIFE, 16 HOURS)...
American Medical Association, AMA Department of Drugs. AMA Drug Evaluations. 5th ed. Chicago: American Medical Association, 1983., p. 779
Imipramine works by inhibiting the neuronal reuptake of the neurotransmitters norepinephrine and serotonin. It binds the sodium-dependent serotonin transporter and sodium-dependent norepinephrine transporter reducing the reuptake of norepinephrine and serotonin by neurons. Depression has been linked to a lack of stimulation of the post-synaptic neuron by norepinephrine and serotonin. Slowing the reuptake of these neurotransmitters increases their concentration in the synaptic cleft, producing knock-on effects in protein kinase signalling which is thought to contribute to changes in neurotransmission and brain physiology which relieves symptoms of depression.
MANNER IN WHICH IMIPRAMINE RELIEVES...DEPRESSION IS NOT CLEAR. ITS EFFECT HAS BEEN DESCRIBED AS DULLING OF DEPRESSIVE IDEATION... HOWEVER, REPORTS OF MANIC EXCITEMENT AS WELL AS EUPHORIA & INSOMNIA INDICATE THAT IMIPRAMINE DOES HAVE STIMULANT ACTION UNDER CERTAIN CIRCUMSTANCES.
Gilman, A. G., L. S. Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 6th ed. New York: Macmillan Publishing Co., Inc. 1980., p. 420
TRICYCLIC ANTIDEPRESSANTS HAVE THREE PRIMARY PHARMACOLOGIC ACTIONS, INCLUDING ANTICHOLINERGIC EFFECTS, REUPTAKE BLOCKADE OF CATECHOLAMINES AT THE ADRENERGIC NEURONAL SITE AND QUINIDINE-LIKE EFFECTS ON THE CARDIAC TISSUE. /TRICYCLIC ANTIDEPRESSANTS/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 983

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
60
PharmaCompass offers a list of Imipramine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Imipramine manufacturer or Imipramine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Imipramine manufacturer or Imipramine supplier.
PharmaCompass also assists you with knowing the Imipramine API Price utilized in the formulation of products. Imipramine API Price is not always fixed or binding as the Imipramine Price is obtained through a variety of data sources. The Imipramine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Imipramine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Imipramine, including repackagers and relabelers. The FDA regulates Imipramine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Imipramine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Imipramine manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Imipramine supplier is an individual or a company that provides Imipramine active pharmaceutical ingredient (API) or Imipramine finished formulations upon request. The Imipramine suppliers may include Imipramine API manufacturers, exporters, distributors and traders.
click here to find a list of Imipramine suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Imipramine DMF (Drug Master File) is a document detailing the whole manufacturing process of Imipramine active pharmaceutical ingredient (API) in detail. Different forms of Imipramine DMFs exist exist since differing nations have different regulations, such as Imipramine USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Imipramine DMF submitted to regulatory agencies in the US is known as a USDMF. Imipramine USDMF includes data on Imipramine's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Imipramine USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Imipramine suppliers with USDMF on PharmaCompass.
A Imipramine written confirmation (Imipramine WC) is an official document issued by a regulatory agency to a Imipramine manufacturer, verifying that the manufacturing facility of a Imipramine active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Imipramine APIs or Imipramine finished pharmaceutical products to another nation, regulatory agencies frequently require a Imipramine WC (written confirmation) as part of the regulatory process.
click here to find a list of Imipramine suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Imipramine as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Imipramine API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Imipramine as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Imipramine and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Imipramine NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Imipramine suppliers with NDC on PharmaCompass.
Imipramine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Imipramine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Imipramine GMP manufacturer or Imipramine GMP API supplier for your needs.
A Imipramine CoA (Certificate of Analysis) is a formal document that attests to Imipramine's compliance with Imipramine specifications and serves as a tool for batch-level quality control.
Imipramine CoA mostly includes findings from lab analyses of a specific batch. For each Imipramine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Imipramine may be tested according to a variety of international standards, such as European Pharmacopoeia (Imipramine EP), Imipramine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Imipramine USP).
