
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
VMF
DRUG PRODUCT COMPOSITIONS
Annual Reports
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
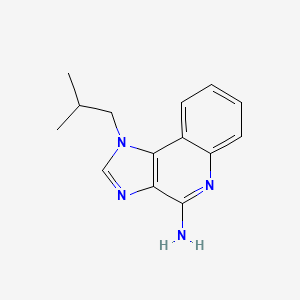

1. 1-isobutyl-1h-imidazo(4,5-c)quinolin-4-amine
2. Aldara
3. R 837
4. R-837
5. R837
6. S 26308
7. S-26308
8. Zyclara
1. 99011-02-6
2. Aldara
3. Zyclara
4. 1-isobutyl-1h-imidazo[4,5-c]quinolin-4-amine
5. 1-(2-methylpropyl)-1h-imidazo[4,5-c]quinolin-4-amine
6. Beselna
7. 4-amino-1-isobutyl-1h-imidazo[4,5-c]quinoline
8. R 837
9. 1-(2-methylpropyl)imidazo[4,5-c]quinolin-4-amine
10. 4-amino-1-isobutyl-1h-imidazo(4,5-c)quinoline
11. 9050-31-1
12. R-837
13. S-26308
14. C14h16n4
15. Mfcd00866946
16. Tmx-101
17. S26308
18. Nsc-369100
19. Nsc-759651
20. Chembl1282
21. 1-isobutylimidazo[4,5-c]quinolin-4-amine
22. P1qw714r7m
23. 1h-imidazo(4,5-c)quinolin-4-amine, 1-(2-methylpropyl)-
24. 1h-imidazo[4,5-c]quinolin-4-amine, 1-(2-methylpropyl)-
25. Chebi:36704
26. Ncgc00070736-02
27. Zartra
28. Imiquimod Acetate
29. Dsstox_cid_21047
30. Dsstox_rid_79617
31. Dsstox_gsid_41047
32. Aldara (tn)
33. Cas-99011-02-6
34. S 26308
35. Sr-01000611320
36. 1-(2-methylpropyl)-1h-imidazole[4,5-c]quinoline-4-amine
37. Unii-p1qw714r7m
38. Imiquimodum
39. Imiquimod [usan:inn:ban]
40. Vyloma
41. Mtd-39
42. Hsdb 8129
43. Tmx 101
44. Imiquimod,(s)
45. Imiquimod- Bio-x
46. 6t0
47. Imiquimod - Aldara
48. Zyclara (tn)
49. Dz-2636
50. R837
51. Imiquimod [inn]
52. Imiquimod [jan]
53. Imiquimod [mi]
54. Imiquimod [usan]
55. Imiquimod [vandf]
56. Imiquimod [mart.]
57. (non-labelled)imiquimod-d9
58. Imiquimod [usp-rs]
59. Imiquimod [who-dd]
60. Imiquimod (jan/usp/inn)
61. Schembl26136
62. Imiquimod [ema Epar]
63. Mls000083577
64. Bidd:gt0859
65. Gtpl5003
66. Imiquimod [orange Book]
67. Dtxsid7041047
68. Imiquimod [usp Monograph]
69. Hms2090m14
70. Hms2232g07
71. Hms3373b13
72. Hms3715n19
73. Hms3747a13
74. Pharmakon1600-01502351
75. Bcp05151
76. Hy-b0180
77. Tox21_110985
78. Ac-529
79. Bbl010772
80. Bdbm50240849
81. Nsc369100
82. Nsc759651
83. Nsc811538
84. S1211
85. Stk583860
86. Zinc19632912
87. Imiquimod - Cas 99011-02-6
88. Imiquimod, >=98% (hplc), Solid
89. Akos005507352
90. Cellulose, Hydrogen 1,2-benzenedicarboxylate, 2-hydroxypropyl Methyl Ether
91. Tox21_110985_1
92. 1h-imidazo[4, 1-(2-methylpropyl)-
93. Ccg-208015
94. Cs-2058
95. Db00724
96. Ks-5218
97. Nsc 369100
98. Nsc 741062
99. Nsc 759651
100. Nsc-811538
101. Yh44175
102. (hydroxypropyl)methyl Cellulose Phthalate
103. Imiquimod 100 Microg/ml In Acetonitrile
104. Ncgc00070736-03
105. Ncgc00070736-04
106. Bi164576
107. Smr000048307
108. Sy017571
109. Ft-0602727
110. I0747
111. D02500
112. 1-isobutyl-1h-imidazo [4,5-c]quinolin-4-amine
113. 1-isobutyl-1h-imidazo[4,5-c]quinoline-4-amine
114. Ab00399298-05
115. Ab00399298-06
116. Ab00399298-07
117. Ab00399298_08
118. Ab00399298_09
119. 011i026
120. 1-isobutyl-1h-imidazo[4,5-c]quinolin-4-ylamine
121. A845945
122. Q423417
123. 1-(2-methylpropyl)-4-imidazo[4,5-c]quinolinamine
124. Sr-01000611320-2
125. Sr-01000611320-3
126. Brd-k26657438-001-01-2
127. Brd-k26657438-001-13-7
128. 1-(2-methylpropyl)-1himidazo[4,5-c]quinolin-4-amine
129. 1-(2-methylpropyl)-1h-imidazo[4,5-c]-quinolin-4-amine
130. Imiquimod, United States Pharmacopeia (usp) Reference Standard
| Molecular Weight | 240.30 g/mol |
|---|---|
| Molecular Formula | C14H16N4 |
| XLogP3 | 2.6 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 2 |
| Exact Mass | 240.137496527 g/mol |
| Monoisotopic Mass | 240.137496527 g/mol |
| Topological Polar Surface Area | 56.7 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 294 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Aldara |
| PubMed Health | Imiquimod (On the skin) |
| Drug Classes | Immune Modulator |
| Drug Label | Aldara (imiquimod 5%) Cream is an immune response modifier for topical administration. Each gram contains 50 mg of imiquimod in an off-white oil-in-water vanishing cream base consisting of isostearic acid, cetyl alcohol, stearyl alcohol, white petrol... |
| Active Ingredient | Imiquimod |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 5% |
| Market Status | Prescription |
| Company | Medicis |
| 2 of 6 | |
|---|---|
| Drug Name | Imiquimod |
| PubMed Health | Imiquimod (On the skin) |
| Drug Classes | Immune Modulator |
| Drug Label | Aldara (imiquimod 5%) Cream is an immune response modifier for topical administration. Each gram contains 50 mg of imiquimod in an off-white oil-in-water vanishing cream base consisting of isostearic acid, cetyl alcohol, stearyl alcohol, white petrol... |
| Active Ingredient | Imiquimod |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 5% |
| Market Status | Prescription |
| Company | Apotex; Teva Pharms Usa; Taro; Strides Pharma; Glenmark Generics; Fougera Pharms; Tolmar; Perrigo Israel |
| 3 of 6 | |
|---|---|
| Drug Name | Zyclara |
| PubMed Health | Imiquimod (On the skin) |
| Drug Classes | Immune Modulator |
| Drug Label | ZYCLARA (imiquimod) Cream, 2.5% or 3.75% is intended for topical administration. Each gram contains 25 mg or 37.5 mg of imiquimod, respectively, in a white to faintly yellow oil-in-water cream base consisting of isostearic acid, cetyl alcohol, steary... |
| Active Ingredient | Imiquimod |
| Dosage Form | Cream |
| Route | topical; Topical |
| Strength | 3.75%; 2.5% |
| Market Status | Prescription |
| Company | Medicis; Graceway |
| 4 of 6 | |
|---|---|
| Drug Name | Aldara |
| PubMed Health | Imiquimod (On the skin) |
| Drug Classes | Immune Modulator |
| Drug Label | Aldara (imiquimod 5%) Cream is an immune response modifier for topical administration. Each gram contains 50 mg of imiquimod in an off-white oil-in-water vanishing cream base consisting of isostearic acid, cetyl alcohol, stearyl alcohol, white petrol... |
| Active Ingredient | Imiquimod |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 5% |
| Market Status | Prescription |
| Company | Medicis |
| 5 of 6 | |
|---|---|
| Drug Name | Imiquimod |
| PubMed Health | Imiquimod (On the skin) |
| Drug Classes | Immune Modulator |
| Drug Label | Aldara (imiquimod 5%) Cream is an immune response modifier for topical administration. Each gram contains 50 mg of imiquimod in an off-white oil-in-water vanishing cream base consisting of isostearic acid, cetyl alcohol, stearyl alcohol, white petrol... |
| Active Ingredient | Imiquimod |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 5% |
| Market Status | Prescription |
| Company | Apotex; Teva Pharms Usa; Taro; Strides Pharma; Glenmark Generics; Fougera Pharms; Tolmar; Perrigo Israel |
| 6 of 6 | |
|---|---|
| Drug Name | Zyclara |
| PubMed Health | Imiquimod (On the skin) |
| Drug Classes | Immune Modulator |
| Drug Label | ZYCLARA (imiquimod) Cream, 2.5% or 3.75% is intended for topical administration. Each gram contains 25 mg or 37.5 mg of imiquimod, respectively, in a white to faintly yellow oil-in-water cream base consisting of isostearic acid, cetyl alcohol, steary... |
| Active Ingredient | Imiquimod |
| Dosage Form | Cream |
| Route | topical; Topical |
| Strength | 3.75%; 2.5% |
| Market Status | Prescription |
| Company | Medicis; Graceway |
Adjuvants, Immunologic; Antineoplastic Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 2013)
Imiquimod is used topically for the treatment of clinically typical, nonhyperkeratotic, nonhypertrophic actinic keratosis on the face or scalp in immunocompetent adults; treatment of biopsy-confirmed, primary superficial basal cell carcinoma in immunocompetent adults; and treatment of external genital and perianal exophytic warts (condylomata acuminata) caused by human papillomavirus (HPV). /Included in US product label/
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 3577
Topical imiquimod has been effective when used in a limited number of adults and children for the treatment of molluscum contagiosum. /NOT included in US product label/
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 3578
Imiquimod 5% cream has been used for the topical treatment of external genital and perianal HPV warts in a limited number of adults with human immunodeficiency virus (HIV) infection; however, the response rate appears to be lower in these individuals than in those who are not HIV infected. /NOT included in US product label/
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 3578
For more Therapeutic Uses (Complete) data for Imiquimod (6 total), please visit the HSDB record page.
Adverse local reactions, including erythema, erosion, excoriation/flaking, and edema, commonly occur at the site of application of imiquimod and/or surrounding areas. These reactions usually are mild to moderate in severity; however, severe local reactions have been reported.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 3578
In controlled studies in adults with actinic keratosis, the most frequently reported local skin reactions in those receiving imiquimod 5% cream (twice weekly for 16 weeks) were erythema (97%), flaking/scaling/dryness (93%), scabbing/crusting (79%), edema (49%), erosion/ulceration (48%), weeping/exudate (22%), and vesicles (9%).1 Application site reactions (e.g., bleeding, burning, induration, irritation, pain, pruritus, stinging, tenderness) occurred in 33% of those receiving topical imiquimod compared with 14% of those receiving placebo. In these studies, 16% of patients discontinued imiquimod treatment because of local or application site reactions and 91% of these were able to resume treatment after a rest period.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 3578
Adverse dermatologic reactions at sites away from the site of application have been reported in some patients receiving topical imiquimod. Remote site reactions have included bleeding, burning, edema, erosion, erythema, excoriation/flaking, induration, pain, pruritus, tenderness, tinea cruris, and ulceration.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 3579
When imiquimod 5% cream was used in controlled studies in patients with genital and perianal HPV warts (3 times weekly for up to 16 weeks), erythema occurred in 58-65%, erosion in 30-31%, excoriation/flaking in 18-26%, edema in 12-18%, scabbing in 4-13%, induration in 5-7%, ulceration in 4-8%, and vesicles in 2-3% of those receiving the drug.1 In addition, application site reactions in those receiving the drug included pruritus (22-32%), burning (9-26%), pain (2-8%), and soreness (0-3%). In addition, fungal infections occurred in 2-11% of patients receiving the drug. Overall, 1.2% of patients in these studies discontinued treatment because of local or application site reactions.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 3579
For more Drug Warnings (Complete) data for Imiquimod (25 total), please visit the HSDB record page.
For the topical treatment of clinically typical, nonhyperkeratotic, nonhypertrophic actinic keratoses on the face or scalp in immunocompetent adults. Also indicated for the treatment of external genital and perianal warts/condyloma acuminata in individuals 12 years old and above.
FDA Label
Zyclara is indicated for the topical treatment of clinically typical, non-hyperkeratotic, non-hypertrophic, visible or palpable actinic keratosis of the full face or balding scalp in immunocompetent adults when other topical treatment options are contraindicated or less appropriate.
Imiquimod cream is indicated for the topical treatment of:
- external genital and perianal warts (condylomata acuminata) in adults;
- small superficial basal-cell carcinomas (sBCCs) in adults;
- clinically typical, non-hyperkeratotic, non-hypertrophic actinic keratoses (AKs) on the face or scalp in immunocompetent adult patients when size or number of lesions limit the efficacy and / or acceptability of cryotherapy and other topical treatment options are contraindicated or less appropriate.
Imiquimod cream is indicated for the topical treatment of external genital and perianal warts (condyloma acuminata) in adult patients.
Imiquimod is an immune response modifier that acts as a toll-like receptor 7 agonist. Imiquimod is commonly used topically to treat warts on the skin of the genital and anal areas. Imiquimod does not cure warts, and new warts may appear during treatment. Imiquimod does not fight the viruses that cause warts directly, however, it does help to relieve and control wart production. It is not used on warts inside the vagina, penis, or rectum. Imiquimod is also used to treat a skin condition of the face and scalp called actinic keratoses. Imiquimod can also be used to treat certain types of skin cancer called superficial basal cell carcinoma. Imiquimod is particularly useful on areas where surgery or other treatments may be difficult, complicated or otherwise undesirable, especially the face and lower legs.
Adjuvants, Immunologic
Substances that augment, stimulate, activate, potentiate, or modulate the immune response at either the cellular or humoral level. The classical agents (Freund's adjuvant, BCG, Corynebacterium parvum, et al.) contain bacterial antigens. Some are endogenous (e.g., histamine, interferon, transfer factor, tuftsin, interleukin-1). Their mode of action is either non-specific, resulting in increased immune responsiveness to a wide variety of antigens, or antigen-specific, i.e., affecting a restricted type of immune response to a narrow group of antigens. The therapeutic efficacy of many biological response modifiers is related to their antigen-specific immunoadjuvanticity. (See all compounds classified as Adjuvants, Immunologic.)
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
Interferon Inducers
Agents that promote the production and release of interferons. They include mitogens, lipopolysaccharides, and the synthetic polymers Poly A-U and Poly I-C. Viruses, bacteria, and protozoa have been also known to induce interferons. (See all compounds classified as Interferon Inducers.)
D06BB10
D06BB10
L03AX
D06BB10
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
D - Dermatologicals
D06 - Antibiotics and chemotherapeutics for dermatological use
D06B - Chemotherapeutics for topical use
D06BB - Antivirals
D06BB10 - Imiquimod
Absorption
Well absorbed through skin (as a cream)
Following topical application to the skin in adults with actinic keratosis (75-mg doses 3 times weekly for 16 weeks), 0.08-0.15% of the dose is eliminated in urine as unchanged drug and metabolites. Following topical application in patients with HPV warts, 0.11 or 2.41% of the dose is eliminated in urine as unchanged drug and metabolites in men or women, respectively.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 3580
Imiquimod is absorbed systemically following topical application to skin. In adults with actinic keratosis who received topical imiquimod 5% cream 3 times weekly for 16 weeks, mean peak serum concentrations at the end of week 16 were approximately 0.1, 0.2, or 3.5 ng/mL in those treated on the face (12.5-mg doses), scalp (25-mg doses), or hands/arms (75-mg doses), respectively. Systemic exposure appeared to depend more on the surface area of the application site than on the total applied dose. In patients with external genital and perianal human papillomavirus (HPV) warts who received topical imiquimod 5% cream (average dose 4.6 mg), mean peak serum concentrations were 0.4 ng/mL.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 3580
20 hours (topical dose), 2 hours (subcutaneous dose)
Studies using subcutaneous imiquimod indicate the drug has an apparent half-life of 2 hours. Following topical application, imiquimod appears to be retained in the skin for prolonged periods since the half-life is approximately 10 times greater than that reported following subcutaneous administration.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 3580
Imiquimod's mechanism of action is via stimulation of innate and acquired immune responses, which ultimately leads to inflammatory cell infiltration within the field of drug application followed by apoptosis of diseased tissue. Imiquimod does not have direct antiviral activity. Studies of mice show that imiquimod may induce cytokines, including interferon-alpha (IFNA) as well as several IFNA genes (IFNA1, IFNA2, IFNA5, IFNA6, and IFNA8) as well as the IFNB gene. Imiquimod also induced the expression of interleukin (IL)-6, IL-8, and tumor necrosis factor alpha genes. In the treatment of basal cell carcinoma, Imiquimod appears to act as a toll-like receptor-7 agonist, and is thought to exert its anti-tumor effect via modification of the immune response and stimulation of apoptosis in BCC cells. In treating basal cell carcinoma it may increase the infiltration of lymphocytes, dendritic cells, and macrophages into the tumor lesion.
Imiquimod and resiquimod represent Toll-like receptor (TLR) 7 and 8 agonists, which emerged as attractive candidates for tumor therapy. To elucidate immune cells, which mainly contribute to TLR7/8-mediated antitumoral activity, /the researchers/ investigated the impact of imiquimod and resiquimod on native human 6-sulfo LacNAc (slan) dendritic cells (DCs). /The researchers/ found that both TLR7/8 agonists significantly improve the release of various proinflammatory cytokines by slanDCs and promote their tumor-directed cytotoxic activity. Furthermore, resiquimod efficiently augmented the ability of slanDCs to stimulate T cells and natural killer cells. These results indicate that imiquimod and resiquimod trigger various immunostimulatory properties of slanDCs, which may contribute to their antitumor effects.
PMID:23402811 Jahnisch H et al; Cancer Lett. 2013 Feb 9 (Epub ahead of print)
Aldara is a cream used for topical treatment of non-melanoma skin cancer, and is thought to act through stimulation of anti-tumour immunity. The active ingredient, imiquimod, has been shown to stimulate toll-like receptor 7. Aldara also induces psoriasis-like lesions when applied to naive murine skin, and as such is used as a mouse model for psoriasis. Here we find that in naive murine skin, Aldara induces inflammation largely independently of toll-like receptor 7. Surprisingly, inflammasome activation, keratinocyte death and interleukin 1 release also occur in response to the vehicle cream in the absence of imiquimod. We show that isostearic acid, a major component of the vehicle, promotes inflammasome activation in cultured keratinocytes, and so may contribute to the observed effects of Aldara on murine skin. Aldara therefore stimulates at least two immune pathways independently, and both imiquimod and vehicle are required for a full inflammatory response. Although it remains to be tested, it is possible that imiquimod-independent effects also contribute to the therapeutic efficacy of Aldara.
PMID:23463003 Walter A et al; Nat Commun. 2013 Mar 5;4:1560
Imiquimod is recognized as an agonist for Toll-like receptor 7 (TLR7) in immunocompetent cells. TLR7, as well as TLR3 and TLR8, triggers the immune responses, such as the production of type I interferons (IFNs) and proinflammatory cytokines via recognition of viral nucleic acids in the infected cells. In this study, /the reseachers/ proposed that imiquimod has an IFN-independent antiviral effect in nonimmune cells. Imiquimod, but not resiquimod, suppressed replication of human herpes simplex virus 1 (HSV-1) in FL cells. We analyzed alternation of gene expression by treatment with imiquimod using microarray analysis. Neither type I IFNs, nor TLRs, nor IFN-inducible antiviral genes were induced in imiquimod-treated FL cells. Cystatin A, a host cysteine protease inhibitor, was strongly upregulated by imiquimod and took a major part in the anti-HSV-1 activity deduced by the suppression experiment using its small interfering RNA. Upregulation of cystatin A was suggested to be mediated by antagonizing adenosine receptor A(1) and activating the protein kinase A pathway. Imiquimod, but not resiquimod, was shown to interact with adenosine receptor A(1). Imiquimod-induced anti-HSV-1 activity was observed in other cells, such as HeLa, SiHa, and CaSki cells, in a manner consistent with the cystatin A induction by imiquimod. These results indicated that imiquimod acted as an antagonist for adenosine receptor A(1) and induced a host antiviral protein, cystatin A. The process occurred independently of TLR7 and type I IFNs.
PMID:22787201 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3457300 Kan Y et al; J Virol 86 (19): 10338-46 (2012)
Toll-like receptor (TLR) agonists have anticancer effect by inducing apoptosis or activating immune cells. In this study, we investigated whether imiquimod, TLR7 agonist, inhibits the proliferation of oral cancer cells. Toll-like receptor 7 expression and IL-6/8 production by imiquimod were examined using RT-PCR and Enzyme-linked immunosorbent assay, respectively. Cell viability was examined by MTT assay. To examine apoptotic cell death, Annexin V/PI staining for flow cytometry and Western blot analysis were performed. Necrotic cell death was determined by leakage of lactate dehydrogenase (LDH), HMGB1, and PI staining in imiquimod-treated oral squamous cell carcinoma (OSCC) cells. Toll-like receptor 7 mRNA was expressed in OSCC cells. Imiquimod induced IL-6 and IL-8 production in OSCC cells, suggesting the functional expression of TLR7. Imiquimod inhibited cells proliferation in a dose-dependent manner. The ratio of annexin V-positive cells and cleaved caspase-3/7 was increased by imiquimod treatment in OSCC cells, suggesting that imiquimod-induced cell death in OSCC cells may be owing to apoptosis. In addition, LDH secretion and PI staining were detected in OSCC cells treated with imiquimod, showing that imiquimod also induced necrotic cell death in the OSCC cells. Imiquimod inhibited effectively the growth of OSCC cells by inducing apoptosis and necrosis.
PMID:22577802 Ahn MY et al; J Oral Pathol Med 41 (7): 540-6 (2012)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
27
PharmaCompass offers a list of Imiquimod API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Imiquimod manufacturer or Imiquimod supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Imiquimod manufacturer or Imiquimod supplier.
PharmaCompass also assists you with knowing the Imiquimod API Price utilized in the formulation of products. Imiquimod API Price is not always fixed or binding as the Imiquimod Price is obtained through a variety of data sources. The Imiquimod Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Imiquimod manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Imiquimod, including repackagers and relabelers. The FDA regulates Imiquimod manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Imiquimod API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Imiquimod manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Imiquimod supplier is an individual or a company that provides Imiquimod active pharmaceutical ingredient (API) or Imiquimod finished formulations upon request. The Imiquimod suppliers may include Imiquimod API manufacturers, exporters, distributors and traders.
click here to find a list of Imiquimod suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Imiquimod DMF (Drug Master File) is a document detailing the whole manufacturing process of Imiquimod active pharmaceutical ingredient (API) in detail. Different forms of Imiquimod DMFs exist exist since differing nations have different regulations, such as Imiquimod USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Imiquimod DMF submitted to regulatory agencies in the US is known as a USDMF. Imiquimod USDMF includes data on Imiquimod's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Imiquimod USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Imiquimod suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Imiquimod Drug Master File in Korea (Imiquimod KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Imiquimod. The MFDS reviews the Imiquimod KDMF as part of the drug registration process and uses the information provided in the Imiquimod KDMF to evaluate the safety and efficacy of the drug.
After submitting a Imiquimod KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Imiquimod API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Imiquimod suppliers with KDMF on PharmaCompass.
A Imiquimod written confirmation (Imiquimod WC) is an official document issued by a regulatory agency to a Imiquimod manufacturer, verifying that the manufacturing facility of a Imiquimod active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Imiquimod APIs or Imiquimod finished pharmaceutical products to another nation, regulatory agencies frequently require a Imiquimod WC (written confirmation) as part of the regulatory process.
click here to find a list of Imiquimod suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Imiquimod as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Imiquimod API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Imiquimod as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Imiquimod and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Imiquimod NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Imiquimod suppliers with NDC on PharmaCompass.
Imiquimod Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Imiquimod GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Imiquimod GMP manufacturer or Imiquimod GMP API supplier for your needs.
A Imiquimod CoA (Certificate of Analysis) is a formal document that attests to Imiquimod's compliance with Imiquimod specifications and serves as a tool for batch-level quality control.
Imiquimod CoA mostly includes findings from lab analyses of a specific batch. For each Imiquimod CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Imiquimod may be tested according to a variety of international standards, such as European Pharmacopoeia (Imiquimod EP), Imiquimod JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Imiquimod USP).
