Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
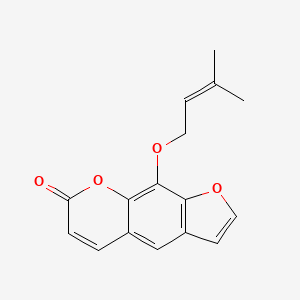

1. 5-hydroxy-8-(1,1-dimethylallyl)psoralen
2. 8-isoamylenoxypsoralen
1. 482-44-0
2. Ammidin
3. Marmelosin
4. Pentosalen
5. 8-isoamylenoxypsoralen
6. 8-isopentenyloxypsoralene
7. Marmelide
8. Nsc 402949
9. 9-(3-methylbut-2-enoxy)furo[3,2-g]chromen-7-one
10. 7h-furo[3,2-g][1]benzopyran-7-one, 9-[(3-methyl-2-butenyl)oxy]-
11. 9-(3-methylbut-2-enyloxy)furo(3,2-g)chromen-7-one
12. Nsc-402949
13. Chebi:5885
14. Chembl453805
15. 9-[(3-methyl-2-buten-1-yl)oxy]-7h-furo[3,2-g]chromen-7-one
16. 9-[(3-methylbut-2-en-1-yl)oxy]-7h-furo[3,2-g]chromen-7-one
17. 9-((3-methyl-2-butenyl)oxy)-7h-furo(3,2-g)(1)benzopyran-7-one
18. Mfcd00016881
19. 9-(3-methylbut-2-enyloxy)furo[3,2-g]chromen-7-one
20. K713n25c78
21. 9-(3-methylbut-2-enyloxy)-7h-furo(3,2-g)chromen-7-one
22. 9-(3-methylbut-2-enyloxy)-7h-furo[3,2-g]chromen-7-one
23. Ncgc00095209-01
24. 9-((3-methylbut-2-en-1-yl)oxy)-7h-furo[3,2-g]chromen-7-one
25. 7h-furo(3,2-g)(1)benzopyran-7-one, 9-((3-methyl-2-butenyl)oxy)-
26. Dsstox_cid_28663
27. Dsstox_rid_82933
28. Dsstox_gsid_48737
29. 9-[(3-methyl-2-butenyl)oxy]-7h-furo[3,2-g]-1-benzopyran-7-one
30. Cas-482-44-0
31. Pentosalen [ban]
32. Ccris 4346
33. Hsdb 3497
34. Einecs 207-581-1
35. Ai3-61725
36. 7h-furo[3,2-g][1]benzopyran-7-one, 9-((3-methyl-2-butenyl)oxy)-
37. Unii-k713n25c78
38. Pentasalen, Ban
39. Imperatorin,(s)
40. 8-prenyloxypsoralen
41. 9-[(3-methyl-2-buten-1-yl)oxy]-7h-furo[3,2-g][1]benzopyran-7-one
42. Spectrum_000755
43. Specplus_000755
44. Imperatorin [mi]
45. Spectrum2_000376
46. Spectrum3_000145
47. Spectrum4_001422
48. Spectrum5_000244
49. Pentosalen [hsdb]
50. [3,2-g]chromen-7-one
51. 6-hydroxy-7-(3-methyl-2-butenyloxy)-5-benzofuranacrylic Acid Omega-lactone
52. 5-benzofuranacrylic Acid, 6-hydroxy-7-((3-methyl-2-butenyl)oxy)-, Delta-lactone
53. Marmelosin [usp-rs]
54. Pentosalen [who-dd]
55. Oprea1_407817
56. Schembl50437
57. Bspbio_001850
58. Kbiogr_001864
59. Kbioss_001235
60. Spectrum102076
61. Mls000574838
62. Divk1c_006851
63. Spbio_000531
64. Marmelosin, Analytical Standard
65. Megxp0_000089
66. Zinc1904
67. Dtxsid8048737
68. Acon1_001117
69. Kbio1_001795
70. Kbio2_001235
71. Kbio2_003803
72. Kbio2_006371
73. Kbio3_001050
74. Hms1922n22
75. Hms2218g08
76. Hms3261c08
77. Hms3354e04
78. Hms3885g06
79. Bcp28280
80. Hy-n0285
81. 8-(3-methyl-2-butenyloxy)psoralen
82. Tox21_113179
83. Tox21_500413
84. Bdbm50308719
85. Ccg-38649
86. Nsc402949
87. S3809
88. 9-(3-methylbut-2-enyloxy)-7h-furo
89. Akos000277029
90. Tox21_113179_1
91. Ac-8046
92. Cs-5800
93. Imperatorin 100 Microg/ml In Methanol
94. Imperatorin, >=98% (hplc), Powder
95. Lp00413
96. Sdccgmls-0066373.p001
97. Sdccgsbi-0052715.p002
98. Ncgc00095209-02
99. Ncgc00095209-03
100. Ncgc00095209-04
101. Ncgc00095209-05
102. Ncgc00095209-06
103. Ncgc00095209-12
104. Ncgc00169661-01
105. Ncgc00169661-02
106. Ncgc00261098-01
107. As-71539
108. Smr000156241
109. Db-050234
110. Wln: T C566 Do Lvotj Bo2uy1&1
111. Ft-0603414
112. I0904
113. N1859
114. 7h-furo[3, 9-[(3-methyl-2-butenyl)oxy]-
115. A14565
116. C09269
117. 482i440
118. A827504
119. Sr-01000721772
120. Q-100532
121. Q1649534
122. Sr-01000721772-2
123. 9-(3-methyl-2-butenyloxy)-7-oxofuro[3,2-g]chromene
124. 9-(3-methyl-but-2-enyloxy)-furo[3,2-g]chromen-7-one
125. Imperatorin, European Pharmacopoeia (ep) Reference Standard
126. 9-(3-methylbut-2-enoxy)furo[3,2-g]chromen-7-one;imperatorin
127. 9-[(3-methyl-2-butenyl)oxy]-7h-furo[3,2-g]chromen-7-one #
128. 9-[(3-methylbut-2-en-1-yl)oxy]-2h-furo[3,2-g]chromen-2-one
129. 9-[(3-methylbut-2-enyl)oxy]-7h-furo[3,2-g][1]benzopyran-7-one
130. (9-[(3-methyl-2-buten-1-yl)oxy]-7h-furo[3,2-g][1]benzopyran-7-one)
131. 7h-furo[3,2-g][1]benzopyran-7-one, 9-[(3-methyl-2-buten-1-yl)oxy]-
132. 9-[(3-methyl-2-butenyl)oxy]-7h-furo[3,2-g][1]benzopyran-7-one, 9ci
133. 5-benzofuranacrylic Acid, 6-hydroxy-7-((3-methyl-2-butenyl)oxy)-, .delta.-lactone
134. 6-hydroxy-7-(3-methyl-2-butenyloxy)-5-benzofuranacrylic Acid .gamma.-lactone
| Molecular Weight | 270.28 g/mol |
|---|---|
| Molecular Formula | C16H14O4 |
| XLogP3 | 3.4 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 3 |
| Exact Mass | 270.08920892 g/mol |
| Monoisotopic Mass | 270.08920892 g/mol |
| Topological Polar Surface Area | 48.7 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 436 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
/EXPL THER/ Several naturally occurring coumarins, to which humans are routinely exposed in the diet, were previously found to inhibit P450-mediated metabolism of benzo[a]pyrene (B[a]P) and 7,12-dimethylbenz[a]anthracene (DMBA) in vitro, block DNA adduct formation in mouse epidermis and inhibit skin tumor initiation by B[a]P and/or DMBA when applied topically to mice. The present study was designed to investigate the effects of two of these compounds, of the linear furanocoumarin type, when given orally (70 mg/kg per os, four successive daily doses), on P450 and glutathione S-transferase (GST) activities and DNA adduct formation by B[a]P and DMBA in various mouse tissues. Imperatorin and isopimpinellin significantly blocked ethoxyresorufin O-deethylase (EROD) and pentoxyresorufin O:-dealkylase (PROD) activities in epidermis at 1 and 24 hr after oral dosing. Imperatorin and isopimpinellin modestly inhibited EROD activities in lung and forestomach at 1 hr and significantly inhibited PROD activities in lung and forestomach at 1 hr after the final oral dose. Twenty-four hours after the final oral dose of imperatorin or isopimpinellin EROD and PROD activities remained inhibited in epidermis and lung. However, forestomach P450 activity had returned to control levels. Interestingly, imperatorin and isopimpinellin treatment inhibited liver EROD activity at 1 hr, had no effect on PROD activity at this time point, but elevated both these enzyme activities at 24 hr. Elevated EROD and PROD activities coincided with elevated hepatic P450 content. Imperatorin and isopimpinellin treatment also increased liver cytosolic GST activity at both 1 and 24 hr after the final oral dose by 1.6-fold compared with corn oil controls. Oral administration of imperatorin and isopimpinellin also had a protective effect against DNA adduct formation by B[a]P and DMBA. Imperatorin pretreatment decreased formation of DNA adducts by DMBA in forestomach. Pretreatment with isopimpinellin led to reduced DNA adduct levels in liver (B[a]P), lung (B[a]P) and mammary epithelial cells (DMBA). These results suggest that imperatorin and isopimpinellin may have potential chemopreventive effects when administered in the diet.
PMID:11159744 Kleiner HE et al; Carcinogenesis 22 (1): 73-82 (2001)
/EXPL THER/ Augmented endothelial nitric oxide (NO) synthase (eNOS) signaling has been reported to be associated with improvements in cardiac remodeling, and NO levels have been shown to be related to cardiac hypertrophy and heart failure. Imperatorin, a dietary furanocoumarin, has been shown to prevent cardiac hypertrophy in the spontaneous hypertension rats (SHR). Thus, we aimed to clarify whether imperatorin attenuates both cardiac hypertrophy and heart failure via the NO-signaling pathway. In neonatal mouse cardiac myocytes, imperatorin inhibited protein synthesis stimulated by either isoproterenol or phenylephrine, which was unchanged by NG-nitro-L-arginine methyl ester (L-NAME). Four weeks after transverse aortic constriction (TAC) on Kunming (KM) male mice, the ratio of heart weight to body weight was lower after imperatorin treatment than in controls (6.60 +/- 0.35 mg/g in TAC, 4.54 +/- 0.29 mg/g with imperatorin 15 mg kg(-1)d(-1), ig, P<0.01); similar changes in the ratio of lung weight to body weight (7.30 +/- 0.85 mg/g in TAC, 5.42 +/- 0.51 mg/g with imperatorin 15 mg/kg/d, ig) and the myocardial fibrosis. All of these improvements were blunted by L-NAME. Imperatorin treatment significantly activated phosphorylation of eNOS. Myocardial mRNA levels of natriuretic peptide precursor type B and protein inhibitor of NO synthase, which were increased in the TAC mice, were decreased in the imperatorin-treated ones. Imperatorin can attenuate cardiac hypertrophy both in vivo and in vitro, and halt the process leading from hypertrophy to heart failure by a NO-mediated pathway.
PMID:21983344 Zhang Y et al; Fitoterapia 83 (1): 60-6 (2012)
/EXPL THER/ The influence of imperatorin (IMP) on the anticonvulsant activity and acute adverse-effect potential of lamotrigine (LTG, a second generation antiepileptic drug) was studied in the maximal electroshock-induced seizure (MES) model and chimney test in mice. In order to assess the nature of interaction between IMP and LTG in the MES test, total brain LTG concentrations were evaluated with high-pressure liquid chromatography (HPLC). Results indicate that IMP administered ip, 30 min before the test, at a dose of 50 mg/kg significantly enhanced the anticonvulsant action of LTG in the MES test by reducing the median effective dose (ED(50)) of LTG from 6.11 to 2.47 mg/kg (p < 0.05). In contrast, IMP administered ip at doses of 30 and 40 mg/kg did not significantly potentiate the anticonvulsant activity of LTG against MES induced seizures, although a reduction of the ED(50) values for LTG from 6.11 to 5.77, and 4.28 mg/kg, respectively, was observed. On the other hand, IMP administered ip, at doses of 30, 40 and 50 mg/kg had no impact on the acute adverse effects of LTG, and the median toxic doses for LTG (TD(50)) were almost unchanged, ranging from 22.13 to 30.04 mg/kg in the chimney test. The protective index (TD(50) to ED(50) ratio) for LTG administered alone was 4.90 and increased to 5.21, 6.77, and 8.96 for LTG in combination with IMPat doses of 30, 40 and 50 mg/kg, respectively. Pharmacokinetic evaluation of total brain LTG concentration with HPLC revealed that IMP at the dose of 50 mg/kg did not affect total brain LTG concentration in experimental animals and thus, the observed interaction between IMP and LTG in the MES test was pharmacodynamic in nature. The present study demonstrates that IMP ameliorates the pharmacological profile of LTG, when considering both, the antiseizure and acute adverse effects of the drug in preclinical study on animals. The combination of LTG with IMP can be of pivotal importance for epileptic patients as a potentially advantageous combination if it is proven that the results of this study can be extrapolated to clinical settings.
PMID:18799827 Luszczki JJ et al; Pharmacol Rep 60 (4): 566-73 (2008)
/EXPL THER/ BACKGROUND: Imperatorin (IM) is a furanocoumarin isolated from the root of Angelica dahurica, which is reported to have anticonvulsant and anticancer effects. In this study, the antiproliferative effect of IM on 9 human cancer cell lines was examined, and human hepatoma HepG2 cells were chosen as the target for preferential killing by IM. Particularly, the mechanism of IM-induced apoptosis and in vivo animal effects were also studied. METHODS: Cell viability was measured using MTT assay, and apoptosis was detected by Hoechst staining, annexin V-PI staining, and DNA laddering assay. Mitochondrial membrane potential was detected by JC-1 staining. Western blot analysis was employed to detect the expression of apoptosis-related proteins. In addition, the in vivo anticancer effect of IM was examined in nude mice bearing HepG2 cells. RESULTS: IM inhibited the proliferation of HepG2 cells through apoptosis induction in a time- and dose-dependent manner by observation of the nuclear morphology, DNA fragmentation, phosphatidylserine externalization, loss of mitochondrial membrane potential, release of cytochrome c into cytosol, and activation of caspase-3, caspase-8, caspase-9, and poly(ADP-ribose) polymerase cleavage. As cell death could partly be prevented by the caspase-8 or caspase-9 inhibitor and was evidenced by the results of Western blot analysis, our results also suggest that IM-induced apoptosis is mediated through both death receptor and mitochondrial pathways. In the animal model, IM was found to effectively suppress tumor growth by 31.93 and 63.18% at dosages of 50 and 100 mg/kg, respectively, after treatment for 14 days. No significant weight loss or toxicity to the hosts was found. CONCLUSIONS: IM can function as a cancer suppressor by inducing apoptosis in HepG2 cells through both death-receptor- and mitochondria-mediated pathways. Furthermore, the in vivo antitumor activities of IM are significant with negligible weight loss and damage to the host.
PMID:22189406 Luo KW et al; Chemotherapy 57 (6): 449-59 (2011)
For more Therapeutic Uses (Complete) data for Imperatorin (9 total), please visit the HSDB record page.
A rapid and sensitive assay for the quantification of imperatorin in plasma and tissues has been developed. An analysis was performed by gas chromatography/mass spectrometry in the selected ion-monitoring mode. The main pharmacokinetic parameters obtained were T(max) = 1.23 +/- 0.26 hr, C(max) = 0.95 +/- 0.38 ug/mL, AUC = 3.42 +/- 0.52 hr ug/mL and K(a) = 1.34 +/- 0.18 hr. The experimental results showed that imperatorin was easily absorbed, but its elimination was slow, from 3 to 12 hr after oral administration. The concentrations of imperatorin in rat liver, kidney, lung, and heart were higher than those in other organs. To determine the free fraction in serum, samples were filtered using ultrafiltration membranes with a molecular weight cut-off of 10 kDa, and extracted using liquid-liquid extraction. The protein binding values in rat plasma, spontaneous hypertensive rat plasma, human plasma and human serum albumin were 84 +/- 3, 69 +/- 7, 81 +/- 7 and 75 +/- 3%, respectively.
PMID:19609025 Wang S et al; Anal Sci 25 (7): 869-73 (2009)
In this study, a simple and sensitive gas chromatography-mass spectrometry method was developed for the study of bioavailability and protein binding and the metabolism of imperatorin in rat. The results showed that the pharmacokinetics of imperatorin after intravenous and oral administration in rats exhibited linear characteristics. The absolute bioavailability of imperatorin was calculated as approximately 3.85, approximately 33.51 and approximately 34.76% for 6.25, 12.5 and 25 mg/kg, respectively. The low bioavailability of imperatorin may be attributed to the poor absorption or extensive metabolism. The phase I metabolites of imperatorin formed in vitro by rat liver microsomes were studied, and two metabolites were isolated and identified as xanthotoxol and heraclenin. Following oral administration of imperatorin, one metabolite (heraclenin) was detected in rat plasma, and two potential metabolites (xanthotoxol and heraclenin) were detected in rat urine. However, none of potential metabolites was detected in rat feces and bile. The results showed that the metabolites of imperatorin were excreted by kidney, and heraclenin was associated with an active component. Demethylation and oxygenization were the main metabolic pathways. In vitro plasma protein binding of imperatorin was 90.1 and 92.6% for the spiked rat plasma concentrations of 1.0 and 50.0 ug/mL, respectively, indicating that imperatorin showed slow distribution into the intra- and extracellular space.
PMID:24357077 Zhao G et al; Biomed Chromatogr 28 (7): 947-56 (2014)
Imperatorin (IMP) is a major constituent of many herbal medicines and possesses anti-osteoporosis activity. The present research work aimed to study the biotransformation processes of IMP and evaluated the anti-osteoporosis activity of the transformed metabolites. Among 18 strains of filamentous fungi screened, Penicillium janthinellum AS 3.510 exhibited good capability to metabolize IMP to the new derivatives. Ten transformed products were isolated and purified, and their structures were identified accurately based on spectroscopic data. Eight metabolites (2-8 and 10) were novel and previously unreported. The major biotransformation reactions involved hydroxylation of the prenyloxy side-chain and the lactone ring-opening reaction of furocoumarin skeleton. In addition, anti-osteoporosis activities of all products (1-10) were evaluated using MC3T3-E1 cells. The results showed that products 5 and 8 had the best bioactivities in increasing MC3T3-E1 cell growth. These products could be used in future therapeutic regimens for treating osteoporosis.
PMID:23497884 Lv X et al; Food Chem 138 (4): 2260-6 (2013)
In this study, a simple and sensitive gas chromatography-mass spectrometry method was developed for the study of bioavailability and protein binding and the metabolism of imperatorin in rat. The results showed that the pharmacokinetics of imperatorin after intravenous and oral administration in rats exhibited linear characteristics. The absolute bioavailability of imperatorin was calculated as approximately 3.85, approximately 33.51 and approximately 34.76% for 6.25, 12.5 and 25 mg/kg, respectively. The low bioavailability of imperatorin may be attributed to the poor absorption or extensive metabolism. The phase I metabolites of imperatorin formed in vitro by rat liver microsomes were studied, and two metabolites were isolated and identified as xanthotoxol and heraclenin. Following oral administration of imperatorin, one metabolite (heraclenin) was detected in rat plasma, and two potential metabolites (xanthotoxol and heraclenin) were detected in rat urine. However, none of potential metabolites was detected in rat feces and bile. The results showed that the metabolites of imperatorin were excreted by kidney, and heraclenin was associated with an active component. Demethylation and oxygenization were the main metabolic pathways. In vitro plasma protein binding of imperatorin was 90.1 and 92.6% for the spiked rat plasma concentrations of 1.0 and 50.0 ug/mL, respectively, indicating that imperatorin showed slow distribution into the intra- and extracellular space.
PMID:24357077 Zhao G et al; Biomed Chromatogr 28 (7): 947-56 (2014)
Imperatorin is a small molecule nature compound isolated from the root of Angelica dahurica, and has been shown to exhibit multiple bioeffector functions, including anti-cancer activity. However, the molecular mechanism underlying imperatorin in suppression of tumor growth is unknown. In this study, we aimed at elucidating the molecular mechanisms underlying imperatorin function and determining the efficacy of imperatorin in suppression of drug-resistant human liver cancer. We observed that imperatorin suppresses tumor cell growth through inducing apoptosis, and imperatorin is more effective in induction of multidrug-resistant human liver cancer cells in vitro. We further determined that imperatorin induces apoptosis through both extrinsic and intrinsic apoptosis pathway. At the molecular level, we identified Mcl-1 as the molecular target of imperatorin and determined that imperatorin induces proteosome-dependent Mcl-1 degradation to release Bak and Bax to trigger apoptosis in liver cancer cells. Consistent with its in vitro apoptosis induction activity, imperatorin exhibited potent activity against multidrug-resistant liver cancer xenograft growth in vivo. Taken together, we determined that imperatorin is a Mcl-1 degradation inducer that can effectively suppress multidrug-resistant human liver cancer growth in vivo, and thus holds great promise for development as an effective small molecule anti-cancer agent in human liver cancer therapy to overcome drug resistance.
PMID:24680709 Li X et al; Cancer Lett 348 (1-2): 146-55 (2014)
Imperatorin, a dietary furocoumarin, is found not only in medicinal plants, but also in popular culinary herbs, such as parsley and fennel. Recently, imperatorin has been shown to activate GPR119 in cells. Another GPR, GPR131, also called TGR5 or G-protein-coupled bile acid receptor 1 (GPBAR1), is known to regulate glucose metabolism. Additionally, TGR5 activation increases glucagon-like peptide (GLP-1) secretion to lower blood sugar levels in animals. Therefore, the present study aims to determine whether the effects of imperatorin on GLP-1 secretion are mediated by TGR5. First, we transfected cultured Chinese hamster ovary cells (CHO-K1 cells) with the TGR5 gene. Glucose uptake was confirmed in the transfected cells using a fluorescent indicator. Moreover, NCI-H716 cells, which secrete GLP-1, were used to investigate the changes in calcium concentrations and GLP-1 levels. In addition, streptozotocin (STZ)-induced type 1-like diabetic rats were used to identify the effects of imperatorin in vivo. Imperatorin dose-dependently increased glucose uptake in CHO-K1 cells expressing TGR5. In STZ diabetic rats, similar to the results in NCI-H716 cells, imperatorin induced a marked increase of GLP-1 secretion that was reduced, but not totally abolished, by a dose of triamterene that inhibited TGR5. Moreover, increases in GLP-1 secretion induced by imperatorin and GPR119 activation were shown in NCI-H716 cells. We demonstrated that imperatorin induced GLP-1 secretion via activating TGR5 and GPR119. Therefore, imperatorin shall be considered as a TGR5 and GPR119 agonist.
PMID:29084156 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5707664 Wang LY et al; Nutrients 9 (11) pii: E1192. doi: 10.3390/nu9111192 (2017)
ABOUT THIS PAGE
25
PharmaCompass offers a list of Imperatorin API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Imperatorin manufacturer or Imperatorin supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Imperatorin manufacturer or Imperatorin supplier.
PharmaCompass also assists you with knowing the Imperatorin API Price utilized in the formulation of products. Imperatorin API Price is not always fixed or binding as the Imperatorin Price is obtained through a variety of data sources. The Imperatorin Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Imperatorin manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Imperatorin, including repackagers and relabelers. The FDA regulates Imperatorin manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Imperatorin API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Imperatorin supplier is an individual or a company that provides Imperatorin active pharmaceutical ingredient (API) or Imperatorin finished formulations upon request. The Imperatorin suppliers may include Imperatorin API manufacturers, exporters, distributors and traders.
Imperatorin Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Imperatorin GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Imperatorin GMP manufacturer or Imperatorin GMP API supplier for your needs.
A Imperatorin CoA (Certificate of Analysis) is a formal document that attests to Imperatorin's compliance with Imperatorin specifications and serves as a tool for batch-level quality control.
Imperatorin CoA mostly includes findings from lab analyses of a specific batch. For each Imperatorin CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Imperatorin may be tested according to a variety of international standards, such as European Pharmacopoeia (Imperatorin EP), Imperatorin JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Imperatorin USP).
