
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Weekly News Recap #Phispers
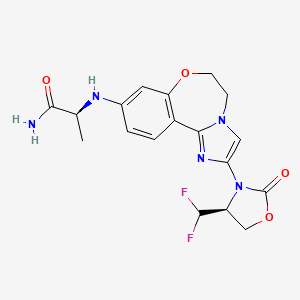

1. (2s)-2-((2-((4s)-4-(difluoromethyl)-2-oxo-1,3-oxazolidin-3-yl)-5,6-dihydroimidazo(1,2-d)(1,4)benzoxazepin-9-yl)amino)propanamide
2. Gdc-0077
1. Gdc-0077
2. 2060571-02-8
3. Rg6114
4. Gdc0077
5. Ro7113755
6. Inavolisib [usan]
7. L4c1uy2nyh
8. (2s)-2-[[2-[(4s)-4-(difluoromethyl)-2-oxo-1,3-oxazolidin-3-yl]-5,6-dihydroimidazo[1,2-d][1,4]benzoxazepin-9-yl]amino]propanamide
9. (s)-2-((2-((s)-4-(difluoromethyl)-2-oxooxazolidin-3-yl)-5,6-dihydrobenzo[f]imidazo[1,2-d][1,4]oxazepin-9-yl)amino)propanamide
10. Rg-6114
11. Ro-7113755
12. N~2~-{(4s,11ap)-2-[(4s)-4-(difluoromethyl)-2-oxo-1,3-oxazolidin-3-yl]-5,6-dihydroimidazo[1,2-d][1,4]benzoxazepin-9-yl}-l-alaninamide
13. Propanamide, 2-((2-((4s)-4-(difluoromethyl)-2-oxo-3-oxazolidinyl)-5,6-dihydroimidazo(1,2-d)(1,4)benzoxazepin-9-yl)amino)-, (2s)-
14. Mfcd31382124
15. Sgeunorsozvtol-cabztgnlsa-n
16. X3n
17. Inavolisib [inn]
18. Unii-l4c1uy2nyh
19. Inavolisib [who-dd]
20. Gtpl9636
21. Chembl4650215
22. Schembl18360780
23. Bdbm295665
24. Amy16810
25. Ex-a2685
26. Nsc800729
27. S8668
28. Us10112932, Compound 101
29. Who 11204
30. Akos040741785
31. At36699
32. Cs-6459
33. Db15275
34. Gdc-0077; Rg6114
35. Nsc-800729
36. Ac-31594
37. Ms-26989
38. Hy-101562
39. A903455
40. Gdc-0077;rg6114;ro-7113755
41. (2s)-2-((2-((4s)-4-(difluoromethyl)-2-oxo-3-oxazolidinyl)-5,6-dihydroimidazo(1,2-d)(1,4)benzoxazepin-9-yl)amino)propanamide
42. (2s)-2-({4-[(4s)-4-(difluoromethyl)-2-oxo-1,3-oxazolidin-3-yl]-9-oxa-3,6-diazatricyclo[8.4.0.0,tetradeca-1(14),2,4,10,12-pentaen-12-yl}amino)propanamide
43. (s)-2-((2-((s)-4-(difluoromethyl)- 2-oxooxazolidin-3-yl)-5,6- Dihydrobenzo[f]imidazo[1,2- D][1,4]oxazepin-9- Yl)amino)propanamide
44. (s)-2-((2-((s)-4-(difluoromethyl)-2-oxooxazolidin-3-yl)-5,6-dihydrobenzo[f]imidazo[1,2-d][1,4]oxazepin-9-yl)propanamide (gdc-0077)
| Molecular Weight | 407.4 g/mol |
|---|---|
| Molecular Formula | C18H19F2N5O4 |
| XLogP3 | 1.5 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 5 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 112 |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 641 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Phosphoinositide-3 Kinase Inhibitors
Agents that inhibit PHOSPHOINOSITIDE-3 KINASE activity. (See all compounds classified as Phosphoinositide-3 Kinase Inhibitors.)
Patents & EXCLUSIVITIES
ABOUT THIS PAGE
