

Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
NDC API
0
VMF
0
FDF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
US Medicaid
NA
Finished Drug Prices
NA
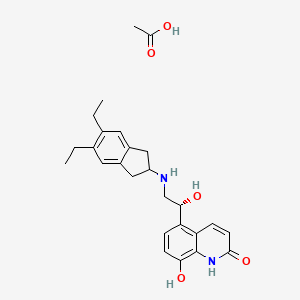

1. 1000160-96-2
2. Ryi4401dtm
3. Indacaterol Acetate (jan)
4. Indacaterol Acetate [jan]
5. Acetic Acid;5-[(1r)-2-[(5,6-diethyl-2,3-dihydro-1h-inden-2-yl)amino]-1-hydroxyethyl]-8-hydroxy-1h-quinolin-2-one
6. Unii-ryi4401dtm
7. Indacaterol Acetic
8. Schembl362019
9. Chembl4297657
10. Dtxsid70142865
11. Ex-a6068
12. Indacaterol Acetate [who-dd]
13. D11009
14. Q27288350
15. (r)-5-[2-(5,6-diethyl-indan-2-ylamino)-1-hydroxyethyl]-8-hydroxy-1h-quinolin-2-one Acetate
| Molecular Weight | 452.5 g/mol |
|---|---|
| Molecular Formula | C26H32N2O5 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 6 |
| Exact Mass | 452.23112213 g/mol |
| Monoisotopic Mass | 452.23112213 g/mol |
| Topological Polar Surface Area | 119 Ų |
| Heavy Atom Count | 33 |
| Formal Charge | 0 |
| Complexity | 620 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
QVM149 combines the bronchodilation of indacaterol acetate (a LABA) and the antimuscarinic effects of glycopyrronium bromide (a LAMA) with mometasone furoate in a precise once-daily formulation, delivered via the dose-confirming Breezhaler® device.
Lead Product(s): Indacaterol Acetate,Glycopyrronium Bromide,Mometasone Furoate
Therapeutic Area: Pulmonary/Respiratory Diseases Brand Name: Enerzair Breezhaler
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Novartis Pharmaceuticals Corporation
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable July 07, 2020
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Indacaterol Acetate,Glycopyrronium Bromide,Mometasone Furoate
Therapeutic Area : Pulmonary/Respiratory Diseases
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Novartis Pharmaceuticals Corporation
Deal Size : Not Applicable
Deal Type : Not Applicable
QVM149 Receives Regulatory Approval in Europe and Japan
Details : QVM149 combines the bronchodilation of indacaterol acetate (a LABA) and the antimuscarinic effects of glycopyrronium bromide (a LAMA) with mometasone furoate in a precise once-daily formulation, delivered via the dose-confirming Breezhaler® device.
Brand Name : Enerzair Breezhaler
Molecule Type : Small molecule
Upfront Cash : Not Applicable
July 07, 2020
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
New post hoc analysis results from the phase III IRIDIUM study that suggests that using Enerzair Breezhaler as a step-up therapy from medium-dose LABA/ICS provides benefit beyond increasing ICS dose alone.
Lead Product(s): Indacaterol Acetate,Glycopyrronium Bromide,Mometasone Furoate
Therapeutic Area: Pulmonary/Respiratory Diseases Brand Name: Enerzair Breezhaler
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable September 03, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Indacaterol Acetate,Glycopyrronium Bromide,Mometasone Furoate
Therapeutic Area : Pulmonary/Respiratory Diseases
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : New post hoc analysis results from the phase III IRIDIUM study that suggests that using Enerzair Breezhaler as a step-up therapy from medium-dose LABA/ICS provides benefit beyond increasing ICS dose alone.
Brand Name : Enerzair Breezhaler
Molecule Type : Small molecule
Upfront Cash : Not Applicable
September 03, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Under the Agreement, Valeo will be responsible for medical and commercial activities for Enerzair® Breezhaler® and Atectura® Breezhaler® for an initial 8 year period.
Lead Product(s): Indacaterol Acetate,Mometasone Furoate
Therapeutic Area: Pulmonary/Respiratory Diseases Brand Name: Atectura Breezhaler
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Valeo Pharma
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Agreement March 29, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Indacaterol Acetate,Mometasone Furoate
Therapeutic Area : Pulmonary/Respiratory Diseases
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Valeo Pharma
Deal Size : Undisclosed
Deal Type : Agreement
Valeo Pharma Inc. Enters into an Agreement for Enerzair® Breezhaler® and Atectura® Breezhaler®...
Details : Under the Agreement, Valeo will be responsible for medical and commercial activities for Enerzair® Breezhaler® and Atectura® Breezhaler® for an initial 8 year period.
Brand Name : Atectura Breezhaler
Molecule Type : Small molecule
Upfront Cash : Undisclosed
March 29, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
High-dose, once-daily Enerzair® Breezhaler® [IND/GLY/MF] reduced asthma exacerbation rates by 21% (moderate or severe) and 31% (severe) versus medium-dose, over 52 weeks.
Lead Product(s): Indacaterol Acetate,Glycopyrronium Bromide,Mometasone Furoate
Therapeutic Area: Pulmonary/Respiratory Diseases Brand Name: Enerzair Breezhaler
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable September 07, 2020

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Indacaterol Acetate,Glycopyrronium Bromide,Mometasone Furoate
Therapeutic Area : Pulmonary/Respiratory Diseases
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Novartis’ phase III IRIDIUM study shows high-dose once-daily Enerzair Breezhaler reduces severe ...
Details : High-dose, once-daily Enerzair® Breezhaler® [IND/GLY/MF] reduced asthma exacerbation rates by 21% (moderate or severe) and 31% (severe) versus medium-dose, over 52 weeks.
Brand Name : Enerzair Breezhaler
Molecule Type : Small molecule
Upfront Cash : Not Applicable
September 07, 2020

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Patients using Enerzair® and Atectura® Breezhaler® to manage their uncontrolled asthma in Japan will have the option to enroll in Propeller’s digital health platform to help manage their condition.
Lead Product(s): Indacaterol Acetate,Glycopyrronium Bromide,Mometasone Furoate
Therapeutic Area: Pulmonary/Respiratory Diseases Brand Name: Enerzair Breezhaler
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Novartis Pharmaceuticals Corporation
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Collaboration August 26, 2020

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Indacaterol Acetate,Glycopyrronium Bromide,Mometasone Furoate
Therapeutic Area : Pulmonary/Respiratory Diseases
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Novartis Pharmaceuticals Corporation
Deal Size : Undisclosed
Deal Type : Collaboration
Propeller Health Enters Japan with Digital Health Sensor for New Enerzair® and Atectura® Breezha...
Details : Patients using Enerzair® and Atectura® Breezhaler® to manage their uncontrolled asthma in Japan will have the option to enroll in Propeller’s digital health platform to help manage their condition.
Brand Name : Enerzair Breezhaler
Molecule Type : Small molecule
Upfront Cash : Undisclosed
August 26, 2020

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
European Commission approves once-daily Enerzair® Breezhaler® in the EU, the first-in-class LABA/LAMA/ICS fixed-dose combination for patients whose asthma is uncontrolled with LABA/ICS1.
Lead Product(s): Indacaterol Acetate,Glycopyrronium Bromide,Mometasone Furoate
Therapeutic Area: Pulmonary/Respiratory Diseases Brand Name: Enerzair Breezhaler
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable July 07, 2020

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Indacaterol Acetate,Glycopyrronium Bromide,Mometasone Furoate
Therapeutic Area : Pulmonary/Respiratory Diseases
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Sosei Heptares Notes Enerzair® Breezhaler® Approval in the European Union as a Treatment for Unc...
Details : European Commission approves once-daily Enerzair® Breezhaler® in the EU, the first-in-class LABA/LAMA/ICS fixed-dose combination for patients whose asthma is uncontrolled with LABA/ICS1.
Brand Name : Enerzair Breezhaler
Molecule Type : Small molecule
Upfront Cash : Not Applicable
July 07, 2020

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
For the first time in Japan, a new digital device combining a sensor with the Breezhaler® inhaler is being made available. Announcement by Novartis Pharma K. K. highlights key milestone for once-daily Enerzair® (IND/GLY/MF) complete with new sensor-enabled Breezhaler® device.
Lead Product(s): Indacaterol Acetate,Glycopyrronium Bromide,Mometasone Furoate
Therapeutic Area: Pulmonary/Respiratory Diseases Brand Name: Enerzair Breezhaler
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Novartis Pharmaceuticals Corporation
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable June 29, 2020

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Indacaterol Acetate,Glycopyrronium Bromide,Mometasone Furoate
Therapeutic Area : Pulmonary/Respiratory Diseases
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Novartis Pharmaceuticals Corporation
Deal Size : Not Applicable
Deal Type : Not Applicable
Sosei Heptares Notes Enerzair® Approval in Japan
Details : For the first time in Japan, a new digital device combining a sensor with the Breezhaler® inhaler is being made available. Announcement by Novartis Pharma K. K. highlights key milestone for once-daily Enerzair® (IND/GLY/MF) complete with new sensor-ena...
Brand Name : Enerzair Breezhaler
Molecule Type : Small molecule
Upfront Cash : Not Applicable
June 29, 2020

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Once-daily Enerzair® Breezhaler® (QVM149; IND/GLY/MF) met primary endpoint, demonstrating non-inferiority to a free combination of twice-daily Sal/Flu plus once-daily Tio in improving quality of life in people with uncontrolled asthma.
Lead Product(s): Indacaterol Acetate,Glycopyrronium Bromide,Mometasone Furoate
Therapeutic Area: Pulmonary/Respiratory Diseases Brand Name: Enerzair Breezhaler
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Novartis Pharmaceuticals Corporation
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable June 05, 2020

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Indacaterol Acetate,Glycopyrronium Bromide,Mometasone Furoate
Therapeutic Area : Pulmonary/Respiratory Diseases
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Novartis Pharmaceuticals Corporation
Deal Size : Not Applicable
Deal Type : Not Applicable
Sosei Heptares Notes Phase IIIb ARGON Study With Enerzair® Breezhaler® (QVM149) Meets Primary En...
Details : Once-daily Enerzair® Breezhaler® (QVM149; IND/GLY/MF) met primary endpoint, demonstrating non-inferiority to a free combination of twice-daily Sal/Flu plus once-daily Tio in improving quality of life in people with uncontrolled asthma.
Brand Name : Enerzair Breezhaler
Molecule Type : Small molecule
Upfront Cash : Not Applicable
June 05, 2020

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Decision supported by robust efficacy and safety data from Phase III IRIDIUM study, in which once-daily Enerzair® Breezhaler® resulted in statistically significant improvements in lung function.
Lead Product(s): Indacaterol Acetate,Glycopyrronium Bromide,Mometasone Furoate
Therapeutic Area: Pulmonary/Respiratory Diseases Brand Name: Enerzair Breezhaler
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Propeller Health
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable May 01, 2020

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Indacaterol Acetate,Glycopyrronium Bromide,Mometasone Furoate
Therapeutic Area : Pulmonary/Respiratory Diseases
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Propeller Health
Deal Size : Not Applicable
Deal Type : Not Applicable
Novartis’ asthma triple heads CHMP’s latest meeting
Details : Decision supported by robust efficacy and safety data from Phase III IRIDIUM study, in which once-daily Enerzair® Breezhaler® resulted in statistically significant improvements in lung function.
Brand Name : Enerzair Breezhaler
Molecule Type : Small molecule
Upfront Cash : Not Applicable
May 01, 2020

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
If the EC follows this recommendation and approves Enerzair® Breezhaler®, it will become the first LABA/long-acting muscarinic antagonist (LAMA)/ICS fixed-dose combination for uncontrolled asthma patients.
Lead Product(s): Indacaterol Acetate,Glycopyrronium Bromide,Mometasone Furoate
Therapeutic Area: Pulmonary/Respiratory Diseases Brand Name: Enerzair Breezhaler
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable May 01, 2020

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Indacaterol Acetate,Glycopyrronium Bromide,Mometasone Furoate
Therapeutic Area : Pulmonary/Respiratory Diseases
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Sosei Heptares Notes that Enerzair® Breezhaler® has Been Recommended for Approval in the E.U for...
Details : If the EC follows this recommendation and approves Enerzair® Breezhaler®, it will become the first LABA/long-acting muscarinic antagonist (LAMA)/ICS fixed-dose combination for uncontrolled asthma patients.
Brand Name : Enerzair Breezhaler
Molecule Type : Small molecule
Upfront Cash : Not Applicable
May 01, 2020

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
06 Sep 2021
// PHARMABIZ

07 Sep 2020
// Selina McKee PHARMATIMES
http://www.pharmatimes.com/news/high-dose_enerzair_breezhaler_further_cuts_asthma_exacerbations_1348771

07 Sep 2020
// PHARMABIZ

10 Jul 2020
// PRESS RELEASE
https://www.novartis.com/news/media-releases/novartis-phase-iii-iridium-data-lancet-respiratory-medicine-show-benefit-enerzair-breezhaler-qvm149-first-class-inhaled-labalamaics-combination-uncontrolled
Global Sales Information

Main Therapeutic Indication : Respiratory Disorders
Currency : USD
2021 Revenue in Millions : 584
2020 Revenue in Millions : 623
Growth (%) : -6

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Market Place

Reply
07 Sep 2021

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
