
Synopsis
Synopsis
0
VMF
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
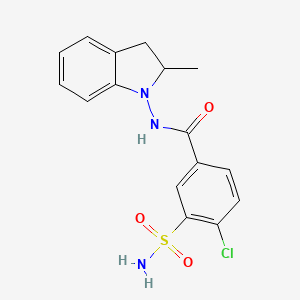

1. Metindamide
2. S 1520
3. S-1520
4. S1520
5. Se 1520
6. Se-1520
7. Se1520
1. 26807-65-8
2. Noranat
3. Veroxil
4. Tertensif
5. Lozol
6. Indaflex
7. Arifon
8. Fludex
9. Indamol
10. Bajaten
11. Ipamix
12. Tandix
13. Natrilix
14. Damide
15. Pressurai
16. Indapamida
17. Indapamidum
18. Natrix
19. Indapamidum [inn-latin]
20. Indapamida [inn-spanish]
21. Se-1520
22. Flupamid
23. Indamide
24. 1-(4-chloro-3-sulfamoylbenzamido)-2-methylindoline
25. Fludin
26. Lorvas
27. 4-chloro-n-(2-methyl-2,3-dihydroindol-1-yl)-3-sulfamoylbenzamide
28. S-1520
29. Benzamide, 3-(aminosulfonyl)-4-chloro-n-(2,3-dihydro-2-methyl-1h-indol-1-yl)-
30. 4-chloro-n-(2-methyl-1-indolinyl)-3-sulfamoylbenzamide
31. Indapamide (lozol)
32. Flubest
33. 4-chloro-n-(2-methyl-2,3-dihydro-1h-indol-1-yl)-3-sulfamoylbenzamide
34. Metindamide
35. Cormil
36. Nsc-757075
37. N-(4-chloro-3-sulfamoylbenzamido)-2-methylindoline
38. Mls000028554
39. Chebi:5893
40. 26807-65-8(free Base)
41. Kyd-041
42. Benzamide, 4-chloro-n-(2-methyl-1-indolinyl)-3-sulfamoyl-
43. F089i0511l
44. Ncgc00018172-03
45. Smr000058829
46. 4-chloro-n-(2-methylindolin-1-yl)-3-sulfamoylbenzamide
47. Dsstox_cid_24633
48. Dsstox_rid_80366
49. Dsstox_gsid_44633
50. Se 1520
51. Rhc 2555
52. Usv 2555
53. Natrix (tn)
54. Lozol (tn)
55. Sr-01000003079
56. Einecs 248-012-7
57. Mfcd00079375
58. Brn 1604026
59. Unii-f089i0511l
60. Hypen Sr
61. 3-(aminosulfonyl)-4-chloro-n-(2,3-dihydro-2-methyl-1h-indol-1-yl)benzamide
62. Indapamide [usan:usp:inn:ban:jan]
63. Spectrum_000917
64. Indapamide [mi]
65. Opera_id_110
66. Indapamide [inn]
67. Indapamide [jan]
68. Prestwick3_000220
69. Spectrum2_000980
70. Spectrum3_000467
71. Spectrum4_000017
72. Spectrum5_000866
73. Indapamide [usan]
74. S1520
75. Indapamide [vandf]
76. Chembl406
77. Indapamide (jp17/usp)
78. Indapamide [mart.]
79. Indapamide [usp-rs]
80. Indapamide [who-dd]
81. Schembl41303
82. Bspbio_000239
83. Bspbio_002174
84. Kbiogr_000393
85. Kbioss_001397
86. 5-20-06-00348 (beilstein Handbook Reference)
87. Mls001148152
88. Mls002222229
89. Mls006011900
90. Divk1c_000508
91. Spectrum1500349
92. Spbio_001019
93. Bpbio1_000263
94. Gtpl7203
95. Dtxsid7044633
96. Indapamide [ep Impurity]
97. Indapamide [orange Book]
98. Bdbm25901
99. Hms501j10
100. Kbio1_000508
101. Kbio2_001397
102. Kbio2_003965
103. Kbio2_006533
104. Kbio3_001394
105. Indapamide [ep Monograph]
106. Ninds_000508
107. Hms1920f19
108. Hms2090m16
109. Hms2091n07
110. Hms2095l21
111. Hms2231g13
112. Hms3259c14
113. Hms3369j20
114. Hms3655e06
115. Hms3712l21
116. Hms3748k05
117. Indapamide [usp Monograph]
118. Pharmakon1600-01500349
119. Indapamide 1.0 Mg/ml In Methanol
120. Amy31945
121. Bcp04140
122. Hy-b0259
123. Tox21_110775
124. Tox21_113182
125. Tox21_302687
126. Ccg-40198
127. Nsc757075
128. S1730
129. Stl257105
130. Stl455045
131. 3-(aminosulfonyl)-4-chloro-n-(2-methyl-2,3-dihydro-1h-indol-1-yl)benzamide
132. Akos015888148
133. Tox21_110775_1
134. Ab03037
135. Ac-2073
136. Db00808
137. Ks-5219
138. Nc00568
139. Nsc 757075
140. Benzamide, 3-(aminosulfonyl)-4-chloro-n-(2,3-dihydro-2-methyl-1h-indol-l-yl)-
141. Idi1_000508
142. Ncgc00018172-02
143. Ncgc00018172-04
144. Ncgc00018172-06
145. Ncgc00018172-08
146. Ncgc00018172-09
147. Ncgc00089727-02
148. Ncgc00089727-03
149. Ncgc00256884-01
150. Sbi-0051415.p003
151. Db-047020
152. Ab00052021
153. I0730
154. Sw198686-2
155. A23574
156. D00345
157. Ab00052021-14
158. Ab00052021-15
159. Ab00052021_16
160. Ab00052021_17
161. 807i658
162. Indapamide, Analytical Standard, For Drug Analysis
163. J-016554
164. Q1078392
165. Sr-01000003079-2
166. Sr-01000003079-3
167. Brd-a95869247-001-04-6
168. Brd-a95869247-001-14-5
169. Z2786158257
170. Indapamide, British Pharmacopoeia (bp) Reference Standard
171. Indapamide, European Pharmacopoeia (ep) Reference Standard
172. Indapamide, United States Pharmacopeia (usp) Reference Standard
173. 3-aminosulfonyl-4-chloro-n-(2,3-dihydro-2-methyl-1h-indol-1-yl)benzamide
174. 4-chloro-3-aminosulphonyl-n-(2,3-dihydro2-methyl-1h-indol-1-yl)benzamide
175. Indapamide, Pharmaceutical Secondary Standard; Certified Reference Material
176. 3-(aminosulphonyl)-4-chloro-n-(2-methyl-2,3-dihydro-1h-indol-1--yl)benzamide
177. 3-(aminosulphonyl)-4-chloro-n-(2-methyl-2,3-dihydro-1h-indol-1-yl)benzamide
178. 4-chloro-n-(2-methyl-2,3-dihydro-1h-indol-1-yl)-3-sulfamoylbenzenecarboximidic Acid
| Molecular Weight | 365.8 g/mol |
|---|---|
| Molecular Formula | C16H16ClN3O3S |
| XLogP3 | 2.9 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 3 |
| Exact Mass | 365.0600902 g/mol |
| Monoisotopic Mass | 365.0600902 g/mol |
| Topological Polar Surface Area | 101 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 580 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Indapamide |
| PubMed Health | Indapamide (By mouth) |
| Drug Classes | Cardiovascular Agent |
| Drug Label | Indapamide is an oral antihypertensive/diuretic. Its molecule contains both a polar sulfamoyl chlorobenzamide moiety and a lipid-soluble methylindoline moiety. It differs chemically from the thiazides in that it does not possess the thiazide ring sys... |
| Active Ingredient | Indapamide |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 2.5mg; 1.25mg |
| Market Status | Prescription |
| Company | Mylan Pharms; Ivax Sub Teva Pharms; Actavis Elizabeth; Mylan |
| 2 of 2 | |
|---|---|
| Drug Name | Indapamide |
| PubMed Health | Indapamide (By mouth) |
| Drug Classes | Cardiovascular Agent |
| Drug Label | Indapamide is an oral antihypertensive/diuretic. Its molecule contains both a polar sulfamoyl chlorobenzamide moiety and a lipid-soluble methylindoline moiety. It differs chemically from the thiazides in that it does not possess the thiazide ring sys... |
| Active Ingredient | Indapamide |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 2.5mg; 1.25mg |
| Market Status | Prescription |
| Company | Mylan Pharms; Ivax Sub Teva Pharms; Actavis Elizabeth; Mylan |
Indapamide is a diuretic indicated for use as monotherapy or in combination with other blood pressure-lowering agents to treat hypertension. It may also be used to treat fluid and salt retention associated with congestive heart failure.
FDA Label
Classified as a sulfonamide diuretic, indapamide is an effective antihypertensive agent and by extension, has shown efficacy in the prevention of target organ damage. Administration of indapamide produces water and electrolyte loss, with higher doses associated with increased diuresis. Severe and clinically significant electrolyte disturbances may occur with indapamide use - for example, hypokalemia resulting from renal potassium loss may lead to QTc prolongation. Further electrolyte imbalances may occur due to renal excretion of sodium, chloride, and magnesium. Other indapamide induced changes include increases in plasma renin and aldosterone, and reduced calcium excretion in the urine. In many studies investigating the effects of indapamide in both non-diabetic and diabetic hypertensive patients, glucose tolerance was not significantly altered. However, additional studies are necessary to assess the long term metabolic impacts of indapamide, since thiazide related impaired glucose tolerance can take several years to develop in non-diabetic patients.
Diuretics
Agents that promote the excretion of urine through their effects on kidney function. (See all compounds classified as Diuretics.)
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
Sodium Chloride Symporter Inhibitors
Agents that inhibit SODIUM CHLORIDE SYMPORTERS. They act as DIURETICS. Excess use is associated with HYPOKALEMIA. (See all compounds classified as Sodium Chloride Symporter Inhibitors.)
C03BA11
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
C - Cardiovascular system
C03 - Diuretics
C03B - Low-ceiling diuretics, excl. thiazides
C03BA - Sulfonamides, plain
C03BA11 - Indapamide
Absorption
The bioavailability of indapamide is virtually complete after an oral dose and is unaffected by food or antacids. Indapamide is highly lipid-soluble due to its indoline moiety - a characteristic that likely explains why indapamides renal clearance makes up less than 10% of its total systemic clearance. The Tmax occurs approximately 2.3 hours after oral administration. The Cmax and AUC0-24 values are 263 ng/mL and 2.95 ug/hr/mL, respectively.
Route of Elimination
An estimated 60-70% of indapamide is eliminated in the urine, while 16-23% is eliminated in the feces.
Volume of Distribution
Some sources report an apparent volume of distribution of 25 L for indapamide, while others report a value of approximately 60 L.
Clearance
Indapamide's renal and hepatic clearance values are reported to be 1.71 mL/min and 20-23.4 mL/min, respectively.
As a result of extensive metabolism in the liver, the majority of indapamide excreted is metabolized, with only 7% remaining unchanged. In humans, as many as 19 distinct indapamide metabolites may be produced, although not all have been identified. There are several metabolic routes through which indapamide may be metabolized, and CYP3A4 is the main enzyme involved in the corresponding hydroxylation, carboxylation, and dehydrogenation reactions. Indapamide can undergo dehydrogenation to form M5, then oxidation to form M4, then further hydroxylation at the indole moiety to form M2. These reactions are facilitated by CYP3A4. Another route of metabolism occurs when indapamide is first hydroxylated to M1 by CYP3A4. M1 then undergoes dehydrogenation to form M3 and is further oxidized to form M2. Hydroxylation of indapamides indole moiety is thought to form the major metabolite (M1), which is less pharmacologically active compared to its parent compound according to animal studies. Indapamide may also undergo epoxidation via CYP3A4 to form a reactive epoxide intermediate. The unstable epoxide intermediate may then undergo dihydroxylation via microsomal epoxide hydrolase to form M6, or glutathione conjugation to form M7.
Indapamide is characterized by biphasic elimination. In healthy subjects, indapamide's elimination half-life can range from 13.9 to 18 hours. The long half-life is conducive to once-daily dosing.
Indapamide acts on the nephron, specifically at the proximal segment of the distal convoluted tubule where it inhibits the Na+/Cl- cotransporter, leading to reduced sodium reabsorption. As a result, sodium and water are retained in the lumen of the nephron for urinary excretion. The effects that follow include reduced plasma volume, reduced venous return, lower cardiac output, and ultimately decreased blood pressure. Interestingly, it is likely that thiazide-like diuretics such as indapamide have additional blood pressure lowering mechanisms that are unrelated to diuresis. This is exemplified by the observation that the antihypertensive effects of thiazides are sustained 4-6 weeks after initiation of therapy, despite recovering plasma and extracellular fluid volumes. Some studies have suggested that indapamide may decrease responsiveness to pressor agents while others have suggested it can decrease peripheral resistance. Although it is clear that diuresis contributes to the antihypertensive effects of indapamide, further studies are needed to investigate the medications ability to decrease peripheral vascular resistance and relax vascular smooth muscle.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
46
PharmaCompass offers a list of Indapamide API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Indapamide manufacturer or Indapamide supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Indapamide manufacturer or Indapamide supplier.
PharmaCompass also assists you with knowing the Indapamide API Price utilized in the formulation of products. Indapamide API Price is not always fixed or binding as the Indapamide Price is obtained through a variety of data sources. The Indapamide Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Indapamide manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Indapamide, including repackagers and relabelers. The FDA regulates Indapamide manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Indapamide API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Indapamide manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Indapamide supplier is an individual or a company that provides Indapamide active pharmaceutical ingredient (API) or Indapamide finished formulations upon request. The Indapamide suppliers may include Indapamide API manufacturers, exporters, distributors and traders.
click here to find a list of Indapamide suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Indapamide DMF (Drug Master File) is a document detailing the whole manufacturing process of Indapamide active pharmaceutical ingredient (API) in detail. Different forms of Indapamide DMFs exist exist since differing nations have different regulations, such as Indapamide USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Indapamide DMF submitted to regulatory agencies in the US is known as a USDMF. Indapamide USDMF includes data on Indapamide's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Indapamide USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Indapamide suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Indapamide Drug Master File in Japan (Indapamide JDMF) empowers Indapamide API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Indapamide JDMF during the approval evaluation for pharmaceutical products. At the time of Indapamide JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Indapamide suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Indapamide Drug Master File in Korea (Indapamide KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Indapamide. The MFDS reviews the Indapamide KDMF as part of the drug registration process and uses the information provided in the Indapamide KDMF to evaluate the safety and efficacy of the drug.
After submitting a Indapamide KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Indapamide API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Indapamide suppliers with KDMF on PharmaCompass.
A Indapamide CEP of the European Pharmacopoeia monograph is often referred to as a Indapamide Certificate of Suitability (COS). The purpose of a Indapamide CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Indapamide EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Indapamide to their clients by showing that a Indapamide CEP has been issued for it. The manufacturer submits a Indapamide CEP (COS) as part of the market authorization procedure, and it takes on the role of a Indapamide CEP holder for the record. Additionally, the data presented in the Indapamide CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Indapamide DMF.
A Indapamide CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Indapamide CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Indapamide suppliers with CEP (COS) on PharmaCompass.
A Indapamide written confirmation (Indapamide WC) is an official document issued by a regulatory agency to a Indapamide manufacturer, verifying that the manufacturing facility of a Indapamide active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Indapamide APIs or Indapamide finished pharmaceutical products to another nation, regulatory agencies frequently require a Indapamide WC (written confirmation) as part of the regulatory process.
click here to find a list of Indapamide suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Indapamide as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Indapamide API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Indapamide as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Indapamide and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Indapamide NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Indapamide suppliers with NDC on PharmaCompass.
Indapamide Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Indapamide GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Indapamide GMP manufacturer or Indapamide GMP API supplier for your needs.
A Indapamide CoA (Certificate of Analysis) is a formal document that attests to Indapamide's compliance with Indapamide specifications and serves as a tool for batch-level quality control.
Indapamide CoA mostly includes findings from lab analyses of a specific batch. For each Indapamide CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Indapamide may be tested according to a variety of international standards, such as European Pharmacopoeia (Indapamide EP), Indapamide JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Indapamide USP).
