



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
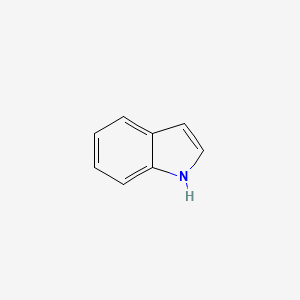

1. Indole Hydrochloride
2. Indole, 14c-labeled
1. 1h-indole
2. 120-72-9
3. 2,3-benzopyrrole
4. Indol
5. 1-benzazole
6. Ketole
7. 1-azaindene
8. Benzopyrrole
9. 2,3-benzopyrole
10. Caswell No. 498b
11. Indol [german]
12. Indole (natural)
13. 1-benzo(b)pyrrole
14. 1h-benzo[b]pyrrole
15. Fema No. 2593
16. Ccris 4421
17. Hsdb 599
18. Epa Pesticide Chemical Code 025000
19. Benzo[b]pyrrole
20. Ai3-01540
21. 1h-indole, Homopolymer
22. Mfcd00005607
23. Chembl15844
24. Chebi:16881
25. 8724fjw4m5
26. Nsc-1964
27. Indole 100 Microg/ml In Acetonitrile
28. Ncgc00167539-01
29. Dsstox_cid_737
30. Dsstox_rid_75761
31. Dsstox_gsid_20737
32. 82451-55-6
33. Ind
34. Cas-120-72-9
35. Nsc 1964
36. Einecs 204-420-7
37. Benzazole
38. Mono-indole
39. Unii-8724fjw4m5
40. 1-h-indole
41. Indole, 7
42. Indolum
43. Indole (8ci)
44. Indole (white Flake)
45. Indole, 98%
46. 1h-indole (9ci)
47. Indolum [hpus]
48. Indole [fhfi]
49. Indole [hsdb]
50. Indole [fcc]
51. Indole [usp-rs]
52. Indole [mi]
53. Indole, >=99%
54. Schembl698
55. Bmse000097
56. Indole, Analytical Standard
57. Indole, >=99%, Fg
58. Wln: T56 Bmj
59. Bidd:gt0304
60. Schembl940818
61. Indole Benzo-pyrrole
62. Schembl1921769
63. Schembl9559244
64. Dtxsid0020737
65. Amy3411
66. Nsc1964
67. 185l
68. Bcp27232
69. Str01201
70. Tox21_112536
71. Tox21_201677
72. Tox21_302937
73. Bbl011739
74. Bdbm50094702
75. S6358
76. Stl163380
77. Zinc14516984
78. Akos000119629
79. Tox21_112536_1
80. Cg-0501
81. Cs-w001132
82. Db04532
83. Hy-w001132
84. Indole, Puriss., >=98.5% (gc)
85. Ncgc00167539-02
86. Ncgc00167539-03
87. Ncgc00256348-01
88. Ncgc00259226-01
89. Bp-10563
90. Ds-011308
91. Ft-0627211
92. I0021
93. C00463
94. I-0800
95. I-0810
96. Q319541
97. Sr-01000944736
98. Sr-01000944736-1
99. Z57833933
100. F2190-0647
| Molecular Weight | 117.15 g/mol |
|---|---|
| Molecular Formula | C8H7N |
| XLogP3 | 2.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 0 |
| Rotatable Bond Count | 0 |
| Exact Mass | 117.057849228 g/mol |
| Monoisotopic Mass | 117.057849228 g/mol |
| Topological Polar Surface Area | 15.8 Ų |
| Heavy Atom Count | 9 |
| Formal Charge | 0 |
| Complexity | 101 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
INDOLE UNDERGOES SCISSION OF PYRROLE RING TO YIELD N-FORMYLANTHRANILIC ACID, AN UNSTABLE COMPD WHICH DECOMP INTO ANTHRANILIC ACID & FORMIC ACID. INDOLE IS FIRST HYDROXYLATED TO GIVE INDOXYL & THEN ISATIN, & IT IS PROBABLY LATTER WHICH UNDERGOES HYDROLYTIC RING OPENING.
Parke, D. V. The Biochemistry of Foreign Compounds. Oxford: Pergamon Press, 1968., p. 69
...INDOLE IS METABOLIZED BY RAT TO INDOXYL, OXINDOLE, 5-HYDROXYOXINDOLE & ISATIN...
Parke, D. V. The Biochemistry of Foreign Compounds. Oxford: Pergamon Press, 1968., p. 45
YIELDS 2,2-BIS(3-INDOLYL)INDOXYL IN HORSERADISH. DEOXYVIOLACEIN IN CHROMOBACTERIUM. CIS-6,7-DIHYDRO-6,7-DIHYDROXYINDOLE PROBABLY IN PSEUDOMONAS. /FROM TABLE/
Goodwin, B.L. Handbook of Intermediary Metabolism of Aromatic Compounds. New York: Wiley, 1976., p. I-2
YIELDS 3,3'-DIINDOLYLACETIC ACID IN PEA. O-FORMAMIDOBENZALDEHYDE IN TECOMA. 3-HYDROXYOXINDOLE IN COCCUS. /FROM TABLE/
Goodwin, B.L. Handbook of Intermediary Metabolism of Aromatic Compounds. New York: Wiley, 1976., p. I-3
For more Metabolism/Metabolites (Complete) data for INDOLE (10 total), please visit the HSDB record page.
Indole has known human metabolites that include 6-Hydroxyindole, Indoxyl, and Oxindole.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
The effects of heterocyclic cmpd on hepatic xenobiotic metabolizing enzymes were studied in mice. Female CD-1 mice were admin 5 u mol/kg coumarin, trimethylene oxide (TMO), or trimethylene sulfide (TMS) for 6 days by gavage, or benzofuran, indole, or indole-3-carbinol (IC) daily for 10 days. Animals were /sacrificed/ 1 or 2 days after the last dose; livers were removed and assayed for epoxide hydrolase, glutathione-S-transferase (GST), reduced NADH-quinone-reductase (NADH/QR), glucose-6-phosphate-dehydrogenase (G6PDH), glutathione-reductase (GSSG-red), uridine-diphosphate-glucose-dehydrogenase (UDPGDH), aniline-hydroxylase, 7-ethoxycoumarin-deethylase (ECOD), and cytochrome-c-reductase (cyt-c-red) activities, and cytochrome p450. All cmpd except indole, significantly enhanced epoxide hydrolase activity. GST activities were elevated by all cmpd except TMO and TMS. NADH/QR activity was incr only by coumarin and benzofuran. Indole incr only GST, UDPGDH and cyt-c-red activities. IC enhanced GST, UDPGDH, cyt-c-red, epoxide hydrolase and cytochrome p450 and related monooxygenase activities. Benzofuran and coumarin showed more varied responses. Both cmpd incr epoxide hydrolase, GST and NADH/QR activities. Benzofuran decr cytochrome p450 content and elevated ECOD activity.
Heine HS et al; Chemico-Biol Interact 59 (2): 219-30 (1986)


