
Synopsis
Synopsis
0
KDMF
0
VMF
Annual Reports
NA
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Amuno
2. Indocid
3. Indocin
4. Indomet 140
5. Indometacin
6. Indomethacin Hydrochloride
7. Metindol
8. Osmosin
1. 53-86-1
2. Indometacin
3. Indocin
4. Indomethacine
5. Indometacine
6. Indocid
7. Metindol
8. Reumacide
9. Indomethazine
10. Imbrilon
11. Amuno
12. Tannex
13. Indomethacinum
14. Artracin
15. Artrinovo
16. Artrivia
17. Confortid
18. Idomethine
19. Indomecol
20. Indoptic
21. Indoptol
22. Inflazon
23. Infrocin
24. Metartril
25. Methazine
26. Mikametan
27. Sadoreum
28. Dolovin
29. Inacid
30. Indacin
31. Indomed
32. Indomee
33. Lausit
34. Metacen
35. Mobilan
36. Indo-rectolmin
37. Indo-tablinen
38. Inteban Sp
39. Durametacin
40. Indometacyna
41. Indometicina
42. Mezolin
43. Indo-lemmon
44. Indometacinum
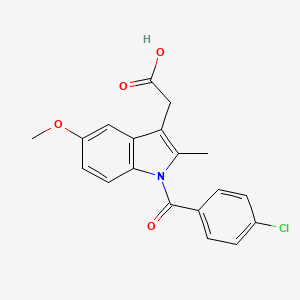
45. 2-(1-(4-chlorobenzoyl)-5-methoxy-2-methyl-1h-indol-3-yl)acetic Acid
46. Indometacina
47. Dolcidium
48. Elmetacin
49. Indomethine
50. Indorektal
51. Aconip
52. Catlep
53. Indoxen
54. Vonum
55. Indo-phlogont
56. Chibro-amuno
57. Rheumacin La
58. 1h-indole-3-acetic Acid, 1-(4-chlorobenzoyl)-5-methoxy-2-methyl-
59. Osmosin
60. Tivorbex
61. 1-(p-chlorobenzoyl)-5-methoxy-2-methylindole-3-acetic Acid
62. Ccris 3502
63. 2-[1-(4-chlorobenzoyl)-5-methoxy-2-methylindol-3-yl]acetic Acid
64. Hsdb 3101
65. 1-(4-chlorobenzoyl)-5-methoxy-2-methyl-3-indoleacetic Acid
66. Nci-c56144
67. Mfcd00057095
68. Indometacin [inn]
69. Chembl6
70. 1-(p-chlorobenzoyl)-2-methyl-5-methoxyindole-3-acetic Acid
71. Imn
72. Indole-3-acetic Acid, 1-(p-chlorobenzoyl)-5-methoxy-2-methyl-
73. Nsc-757061
74. 1-(4-chlorobenzoyl)-5-methoxy-2-methyl-1h-indole-3-acetic Acid
75. [1-(4-chlorobenzoyl)-5-methoxy-2-methyl-1h-indol-3-yl]acetic Acid
76. Indocin Sr
77. Xxe1cet956
78. 2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl-1h-indol-3-yl}acetic Acid
79. N-p-chlorbenzoyl-5-methoxy-2-methylindole-3-acetic Acid
80. Mls000069402
81. Indomethacin (indocid, Indocin)
82. (1-p-chlorobenzoyl-5-methoxy-2-methylindol-3-yl)acetic Acid
83. Chebi:49662
84. 1-(p-chlorobenzoyl)-2-methoxy-3-methyl-1h-indole-3-acetic Acid
85. Indomet 140
86. Alpha-(1-(p-chlorobenzoyl)-2-methyl-5-methoxy-3-indolyl)acetic Acid
87. {1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl-1h-indol-3-yl}acetic Acid
88. Nsc-77541
89. Cas-53-86-1
90. 1-p-cloro-benzoil-5-metoxi-2-metilindol-3-acido Acetico
91. Ncgc00015562-18
92. Indmethacine
93. Indomethancin
94. 1-(p-chlorobenzoyl)-5-methoxy-2-methyl-3-indolylacetic Acid
95. Arthrexin
96. Bonidin
97. Bonidon
98. Indameth
99. Indomod
100. Miametan
101. Smr000058195
102. Indomo
103. Flexin Continus
104. Hicin
105. Kwas 1-(p-chlorobenzoilo)-2-metylo-5-metoksy-3-indolilooctowy
106. Chrono-indicid
107. Chrono-indocid
108. Indometacyna [polish]
109. Dsstox_cid_740
110. Bonidon Gel
111. Indometicina [spanish]
112. 2-[1-(4-chlorobenzoyl)-5-methoxy-2-methyl-1h-indol-3-yl]acetic Acid
113. Dolcidium Pl
114. Indo-spray
115. Indolar Sr
116. Dsstox_rid_75763
117. Dsstox_gsid_20740
118. Indometacine [inn-french]
119. Indometacinum [inn-latin]
120. Indometacina [inn-spanish]
121. 1-(4-chlorobenzoyl)-5-methoxy-2-methylindole-3-acetic Acid
122. 2-[1-(4-chlorobenzoyl)-5-methoxy-2-methyl-indol-3-yl]acetic Acid
123. 2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methylindol-3-yl}acetic Acid
124. Indocin-sr
125. Indochron E-r
126. Indocin (tn)
127. Aconip (tn)
128. Indomethacin (usp)
129. Flam
130. Indocid (pharmaceutical)
131. Sr-01000000014
132. Einecs 200-186-5
133. Indomethacin & Map-30
134. Indomethacin [usan:usp]
135. Unii-xxe1cet956
136. Brn 0497341
137. Indocollyre
138. Indonol
139. Innamit
140. Inteban
141. (1-(4-chlorobenzoyl)-5-methoxy-2-methyl-1h-indol-3-yl)acetic Acid
142. 2-{1-((4-chlorophenyl)carbonyl)-5-methoxy-2-methylindol-3-yl}acetic Acid
143. 4kyk
144. Indomethacin,(s)
145. Prestwick_597
146. Opera_id_56
147. Spectrum_000919
148. Tocris-1708
149. 1z9h
150. 1-(p-chlorbenzoyl)-5-methoxy-2-methylindol-3-essigsaeure [german]
151. 1-p-cloro-benzoil-5-metoxi-2-metilindol-3-acido Acetico [spanish]
152. Prestwick0_000272
153. Prestwick1_000272
154. Prestwick2_000272
155. Prestwick3_000272
156. Spectrum2_000970
157. Spectrum3_000468
158. Spectrum4_000018
159. Spectrum5_000868
160. Indometacin [jan]
161. Indomethacin [mi]
162. Lopac-i-7378
163. Kwas 1-(p-chlorobenzoilo)-2-metylo-5-metoksy-3-indolilooctowy [polish]
164. Molmap_000032
165. Upcmld-dp023
166. Ec 200-186-5
167. I 7378
168. Indometacin (jp17/inn)
169. Indomethacin [hsdb]
170. Indomethacin [usan]
171. Schembl9300
172. Indometacin [mart.]
173. Indomethacin [vandf]
174. Lopac0_000692
175. Oprea1_686105
176. Bspbio_000144
177. Bspbio_001149
178. Bspbio_002176
179. Indometacin [who-dd]
180. Indometacin [who-ip]
181. Indomethacine Impurity Mixture
182. Kbiogr_000395
183. Kbiogr_000489
184. Kbioss_000489
185. Kbioss_001399
186. 5-22-05-00239 (beilstein Handbook Reference)
187. Mls001074194
188. Mls006011845
189. Bidd:gt0132
190. Divk1c_000271
191. Indomethacin [usp-rs]
192. Spectrum1500350
193. Spbio_000979
194. Spbio_002363
195. Bpbio1_000160
196. Gtpl1909
197. Dtxsid9020740
198. Indomethacin, >=99% (tlc)
199. Upcmld-dp023:001
200. Bdbm17638
201. Cgigdmfjxjatdk-uhfffaoysa-
202. Hms500n13
203. Kbio1_000271
204. Kbio2_000489
205. Kbio2_001399
206. Kbio2_003057
207. Kbio2_003967
208. Kbio2_005625
209. Kbio2_006535
210. Kbio3_000897
211. Kbio3_000898
212. Kbio3_001396
213. 1-(p-chlorbenzoyl)-5-methoxy-2-methylindol-3-essigsaeure
214. Indomethacin - Cas 53-86-1
215. Ninds_000271
216. Bio2_000405
217. Bio2_000885
218. Hms1362i11
219. Hms1568h06
220. Hms1792i11
221. Hms1920f21
222. Hms1990i11
223. Hms2089n19
224. Hms2091n09
225. Hms2095h06
226. Hms2231j10
227. Hms3262k05
228. Hms3268a14
229. Hms3374f07
230. Hms3403i11
231. Hms3414n13
232. Hms3430l03
233. Hms3649k17
234. Hms3655o04
235. Hms3678n11
236. Hms3712h06
237. Hms3747k21
238. Hms3884e08
239. Indometacin [ep Monograph]
240. Indomethacin [orange Book]
241. Indomethacin-d4(chlorobenzoyl-d4)
242. Pharmakon1600-01500350
243. Zinc601283
244. Act02579
245. Bcp18951
246. Indomethacin, >=99.0% (tlc)
247. Indomethacin [usp Monograph]
248. Tox21_113109
249. Tox21_201791
250. Tox21_300266
251. Tox21_500692
252. Ac-532
253. Ccg-40186
254. Indometacinum [who-ip Latin]
255. Nsc757061
256. S1723
257. Stl257874
258. Akos000592893
259. Tox21_113109_1
260. At13679
261. Db00328
262. Indometacin 1.0 Mg/ml In Acetonitrile
263. Ks-5255
264. Lp00692
265. Nsc 757061
266. Sdccgsbi-0050670.p005
267. Idi1_000271
268. Idi1_002160
269. Ncgc00015562-01
270. Ncgc00015562-02
271. Ncgc00015562-03
272. Ncgc00015562-04
273. Ncgc00015562-05
274. Ncgc00015562-06
275. Ncgc00015562-07
276. Ncgc00015562-08
277. Ncgc00015562-09
278. Ncgc00015562-10
279. Ncgc00015562-11
280. Ncgc00015562-12
281. Ncgc00015562-13
282. Ncgc00015562-14
283. Ncgc00015562-15
284. Ncgc00015562-16
285. Ncgc00015562-17
286. Ncgc00015562-19
287. Ncgc00015562-20
288. Ncgc00015562-21
289. Ncgc00015562-22
290. Ncgc00015562-24
291. Ncgc00015562-25
292. Ncgc00015562-40
293. Ncgc00024135-02
294. Ncgc00024135-04
295. Ncgc00024135-05
296. Ncgc00024135-06
297. Ncgc00024135-07
298. Ncgc00024135-08
299. Ncgc00024135-09
300. Ncgc00024135-10
301. Ncgc00024135-11
302. Ncgc00024135-12
303. Ncgc00024135-13
304. Ncgc00024135-14
305. Ncgc00024135-15
306. Ncgc00254075-01
307. Ncgc00259340-01
308. Ncgc00261377-01
309. Bi166166
310. Bp-30207
311. Hy-14397
312. Nci60_041708
313. Sbi-0050670.p004
314. Acemetacin Impurity B [ep Impurity]
315. Db-052413
316. Ab00052022
317. Eu-0100692
318. Ft-0603227
319. I0655
320. Indomethacine 100 Microg/ml In Acetonitrile
321. Sw196768-5
322. Indomethacin, Meets Usp Testing Specifications
323. Bim-0050670.0001
324. C01926
325. D00141
326. S00108
327. Ab00052022-20
328. Ab00052022-21
329. Ab00052022_23
330. Ab00052022_24
331. L000959
332. Q409231
333. Indomethacin, Antibiotic For Culture Media Use Only
334. Q-201239
335. Sr-01000000014-2
336. Sr-01000000014-4
337. Sr-01000000014-6
338. Brd-k57222227-001-06-1
339. Brd-k57222227-001-18-6
340. Brd-k57222227-001-27-7
341. Sr-01000000014-10
342. Sr-01000000014-16
343. Z56784896
344. 1-p-chlorobenzoyl-2-methyl-5-methoxyindol-3-acetic Acid
345. 1-(p-chlorobenzoyl)-2-methyl-5-methoxy-3-indoleacetic Acid
346. 1-(p-chlorobenzoyl)-5-methoxy-2-methyl-3-indoleacetic Acid
347. 1-(p-chlorobenzoyl)-5-methoxy-2-methylindol-3-acetic Acid
348. 1-(4-chloro-benzoyl)-5-methoxy-2-methyl-3-indolyl-acetic Acid
349. 1-(4-chlorobenzoyl)-2-methyl-5-methoxyindole-3-acetic Acid
350. 1-(p-chlorobenzoyl)-2-methyl-5-methoxy-3-indole-acetic Acid
351. 1-(p-chlorobenzoyl)-2-methyl-5-methoxy-3-indolylacetic Acid
352. 1-(p-chlorobenzoyl)-5-methoxy-2-methyl-3-indol Acetic Acid
353. 1-(p-chlorobenzoyl)-5-methoxy-2-methyl-indole-3-acetic Acid
354. Indomethacin, European Pharmacopoeia (ep) Reference Standard
355. N-(p-chlorobenzoyl)-2-methyl-5-methoxy-3-indolylacetic Acid
356. Indomethacin, United States Pharmacopeia (usp) Reference Standard
357. .alpha.-(1-(p-chlorobenzoyl)-2-methyl-5-methoxy-3-indolyl)acetic Acid
358. [1-(4-chlorobenzoyl)-5-methoxy-2-methyl-1h-indol-3-yl]acetic Acid #
359. 1-(4-chlorobenzoyl)-5-methoxy-2-methyl-1h-indole-3-acetic Acid & Map-30
360. 1h-indole-3-acetic Acid, 1-(4-chlorobenzoyl)-5-methoxy-2-methyl- (9ci)
361. 2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2 Methylindol-3-yl}acetic Acid
362. 2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2methylindol-3-yl}acetic Acid
363. Indole-3-acetic Acid, 1-(p-chlorobenzoyl)-5-methoxy-2-methyl- (8ci)
364. Indomethacin, Pharmaceutical Secondary Standard; Certified Reference Material
| Molecular Weight | 357.8 g/mol |
|---|---|
| Molecular Formula | C19H16ClNO4 |
| XLogP3 | 4.3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 4 |
| Exact Mass | 357.0767857 g/mol |
| Monoisotopic Mass | 357.0767857 g/mol |
| Topological Polar Surface Area | 68.5 Ų |
| Heavy Atom Count | 25 |
| Formal Charge | 0 |
| Complexity | 506 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Indocin |
| PubMed Health | Indomethacin (Injection) |
| Drug Classes | Analgesic, Central Nervous System Agent |
| Drug Label | Suspension INDOCINRegistered trademark of MERCK & CO., Inc., Whitehouse Station, NJ U.S.A. and licensed to Iroko Pharmaceuticals, LLC, Philadelphia, PA, U.S.A. All rights reserved for oral use contains 25mg of indomethacin per 5mL, alcohol 1%, an... |
| Active Ingredient | Indomethacin sodium; Indomethacin |
| Dosage Form | Suspension; Injectable |
| Route | Injection; Oral |
| Strength | eq 1mg base/vial; 25mg/5ml |
| Market Status | Prescription |
| Company | Recordati Rare; Iroko Pharms |
| 2 of 4 | |
|---|---|
| Drug Name | Indomethacin |
| PubMed Health | Indomethacin |
| Drug Classes | Acetic Acid |
| Drug Label | Indomethacin cannot be considered a simple analgesic and should not be used in conditions other than those recommended underINDICATION AND USAGE.Indomethacin is a non-steroidal anti-inflammatory indole derivative designated chemically as 1-(4-chlorob... |
| Active Ingredient | Indomethacin |
| Dosage Form | Capsule; Suppository; Injectable; Capsule, extended release |
| Route | Rectal; Injection; Oral |
| Strength | eq 1mg base/vial; 25mg; 75mg; 50mg |
| Market Status | Prescription |
| Company | Mylan Pharms; Amneal Pharms; Avanthi; Hetero Labs Ltd Iii; Sun Pharm Inds; Sandoz; Watson Labs; Glenmark Generics; Ivax Sub Teva Pharms; Paddock; Fresenius Kabi Usa; Zydus Pharms Usa; G And W Labs; Mylan; Heritage Pharms |
| 3 of 4 | |
|---|---|
| Drug Name | Indocin |
| PubMed Health | Indomethacin (Injection) |
| Drug Classes | Analgesic, Central Nervous System Agent |
| Drug Label | Suspension INDOCINRegistered trademark of MERCK & CO., Inc., Whitehouse Station, NJ U.S.A. and licensed to Iroko Pharmaceuticals, LLC, Philadelphia, PA, U.S.A. All rights reserved for oral use contains 25mg of indomethacin per 5mL, alcohol 1%, an... |
| Active Ingredient | Indomethacin sodium; Indomethacin |
| Dosage Form | Suspension; Injectable |
| Route | Injection; Oral |
| Strength | eq 1mg base/vial; 25mg/5ml |
| Market Status | Prescription |
| Company | Recordati Rare; Iroko Pharms |
| 4 of 4 | |
|---|---|
| Drug Name | Indomethacin |
| PubMed Health | Indomethacin |
| Drug Classes | Acetic Acid |
| Drug Label | Indomethacin cannot be considered a simple analgesic and should not be used in conditions other than those recommended underINDICATION AND USAGE.Indomethacin is a non-steroidal anti-inflammatory indole derivative designated chemically as 1-(4-chlorob... |
| Active Ingredient | Indomethacin |
| Dosage Form | Capsule; Suppository; Injectable; Capsule, extended release |
| Route | Rectal; Injection; Oral |
| Strength | eq 1mg base/vial; 25mg; 75mg; 50mg |
| Market Status | Prescription |
| Company | Mylan Pharms; Amneal Pharms; Avanthi; Hetero Labs Ltd Iii; Sun Pharm Inds; Sandoz; Watson Labs; Glenmark Generics; Ivax Sub Teva Pharms; Paddock; Fresenius Kabi Usa; Zydus Pharms Usa; G And W Labs; Mylan; Heritage Pharms |
Anti-Inflammatory Agents, Non-Steroidal; Cardiovascular Agents; Cyclooxygenase Inhibitors; Gout Suppressants; Tocolytic Agents
National Library of Medicine's Medical Subject Headings. Indomethacin. Online file (MeSH, 2016). Available from, as of January 20, 2016: https://www.nlm.nih.gov/mesh/2016/mesh_browser/MBrowser.html
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Indomethacin is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of March 17, 2016: https://clinicaltrials.gov/ct2/results?term=indomethacin&Search=Search
Carefully consider the potential benefits and risks of indomethacin capsules and other treatment options before deciding to use indomethacin. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals. Indomethacin has been found effective in active stages of the following: Moderate to severe rheumatoid arthritis including acute flares of chronic disease. Moderate to severe ankylosing spondylitis. Moderate to severe osteoarthritis. Acute painful shoulder (bursitis and/or tendinitis). Acute gouty arthritis. /Included in US product labeling/
NIH; DailyMed. Current Medication Information for Indomethacin Capsule (Updated: August 12, 2015). Available from, as of March 28, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6422d983-a063-41ae-aa96-7ae2284cb123
Indomethacin for Injection is indicated to close a hemodynamically significant patent ductus arteriosus in premature infants weighing between 500 and 1,750 g when 48 hours usual medical management (e.g., fluid restriction, diuretics, digitalis, respiratory support, etc.) is ineffective. Clear-cut clinical evidence of a hemodynamically significant patent ductus arteriosus should be present, such as respiratory distress, a continuous murmur, a hyperactive precordium, cardiomegaly, or pulmonary plethora on chest x-ray. /Included in US product label/
NIH; DailyMed. Current Medication Information for Indomethacin Injection, Powder, Lyophilized, for Solution (Updated: April 29, 2014). Available from, as of March 28, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=30e38747-c553-4e2d-bc23-634e1dd90b8d
For more Therapeutic Uses (Complete) data for INDOMETHACIN (19 total), please visit the HSDB record page.
/BOXED WARNING/ Cardiovascular Risk. Nonsteroidal anti-inflammatory drugs (NSAIDs) may cause an increased risk of serious cardiovascular thrombotic events, myocardial infarction, and stroke, which can be fatal. This risk may increase with duration of use. Patients with cardiovascular disease or risk factors for cardiovascular disease may be at greater risk. Indomethacin is contraindicated for the treatment of perioperative pain in the setting of coronary artery bypass graft (CABG) surgery.
NIH; DailyMed. Current Medication Information for Indomethacin Capsule (Updated: August 12, 2015). Available from, as of March 28, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6422d983-a063-41ae-aa96-7ae2284cb123
/BOXED WARNING/ Gastrointestinal Risk. Nonsteroidal anti-inflammatory drugs (NSAIDs) cause an increased risk of serious gastrointestinal adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients are at greater risk for serious gastrointestinal events.
NIH; DailyMed. Current Medication Information for Indomethacin Capsule (Updated: August 12, 2015). Available from, as of March 28, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6422d983-a063-41ae-aa96-7ae2284cb123
Indomethacin should be used with extreme caution and under close supervision in patients with a history of GI bleeding or peptic ulcer disease, and such patients should receive an appropriate ulcer preventive regimen. All patients considered at increased risk of potentially serious adverse GI effects (e.g., geriatric patients, those receiving high therapeutic dosages of NSAIAs, those with a history of peptic ulcer disease, those receiving anticoagulants or corticosteroids concomitantly) should be monitored closely for signs of ulcer perforation or GI bleeding. To minimize the potential risk of adverse GI effects, the lowest effective dosage and shortest possible duration of therapy should be employed. For patients who are at high risk, therapy other than an NSAIA should be considered.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2083
Adverse dermatologic effects of indomethacin occur in less than 1% of patients and include pruritus, urticaria, rash, macular and morbilliform eruptions, erythema nodosum, petechiae or ecchymosis, exfoliative dermatitis, loss of hair, Stevens-Johnson syndrome, erythema multiforme, and toxic epidermal necrolysis.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2082
For more Drug Warnings (Complete) data for INDOMETHACIN (43 total), please visit the HSDB record page.
Oral indometacin is indicated for symptomatic management of moderate to severe rheumatoid arthritis including acute flares of chronic disease, moderate to severe ankylosing spondylitis, moderate to severe osteoarthritis, acute painful shoulder (bursitis and/or tendinitis) and acute gouty arthritis. Intravenous indometacin is indicated to induce closure of a hemodynamically significant patent ductus arteriosus in premature infants weighing between 500 and 1750 g when after 48 hours usual medical management (e.g., fluid restriction, diuretics, digitalis, respiratory support, etc.) is ineffective.
Indometacin is an NSAID with analgesic and antipyretic properties that exerts its pharmacological effects by inhibiting the synthesis of factors involved in pain, fever, and inflammation. Its therapeutic action does not involve pituitary-adrenal stimulation. Indometacin primarily works by suppressing inflammation in rheumatoid arthritis by providing relief of pain as well as reducing fever, swelling, and tenderness. This effectiveness has been demonstrated by a reduction in the extent of joint swelling, the average number of joints displaying symptoms of inflammation, and the severity of morning stiffness. Increased mobility was demonstrated by a decrease in total walking time and by improved functional capability seen as an increase in grip strength. In clinical trials, indometacin was shown to be effective in relieving the pain, reducing the fever, swelling, redness, and tenderness of acute gouty arthritis. Due to its pharmacological actions, the use of indometacin is associated with the risk of serious cardiovascular thrombotic events, including myocardial infarction and stroke, as well as gastrointestinal effects such as bleeding, ulceration, and perforation of the stomach or intestines. In a study of healthy individuals, acute oral and intravenous indometacin therapy resulted in a transiently diminished basal and CO2 stimulated cerebral blood flow; this effect disappeared in one study after one week of oral treatment. The clinical significance of this effect has not been established. Compared to other NSAIDs, it is suggested that indometacin is a more potent vasoconstrictor that is more consistent in decreasing cerebral blood flow and inhibiting CO2 reactivity. There have been studies that show indometacin directly inhibiting neuronal activity to some extent in the trigeminocervical complex after either superior salivatory nucleus or dural stimulation.
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
Cardiovascular Agents
Agents that affect the rate or intensity of cardiac contraction, blood vessel diameter, or blood volume. (See all compounds classified as Cardiovascular Agents.)
Tocolytic Agents
Drugs that prevent preterm labor and immature birth by suppressing uterine contractions (TOCOLYSIS). Agents used to delay premature uterine activity include magnesium sulfate, beta-mimetics, oxytocin antagonists, calcium channel inhibitors, and adrenergic beta-receptor agonists. The use of intravenous alcohol as a tocolytic is now obsolete. (See all compounds classified as Tocolytic Agents.)
Cyclooxygenase Inhibitors
Compounds or agents that combine with cyclooxygenase (PROSTAGLANDIN-ENDOPEROXIDE SYNTHASES) and thereby prevent its substrate-enzyme combination with arachidonic acid and the formation of eicosanoids, prostaglandins, and thromboxanes. (See all compounds classified as Cyclooxygenase Inhibitors.)
Gout Suppressants
Agents that increase uric acid excretion by the kidney (URICOSURIC AGENTS), decrease uric acid production (antihyperuricemics), or alleviate the pain and inflammation of acute attacks of gout. (See all compounds classified as Gout Suppressants.)
M01AB01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
C - Cardiovascular system
C01 - Cardiac therapy
C01E - Other cardiac preparations
C01EB - Other cardiac preparations
C01EB03 - Indometacin
M - Musculo-skeletal system
M01 - Antiinflammatory and antirheumatic products
M01A - Antiinflammatory and antirheumatic products, non-steroids
M01AB - Acetic acid derivatives and related substances
M01AB01 - Indometacin
M - Musculo-skeletal system
M02 - Topical products for joint and muscular pain
M02A - Topical products for joint and muscular pain
M02AA - Antiinflammatory preparations, non-steroids for topical use
M02AA23 - Indometacin
S - Sensory organs
S01 - Ophthalmologicals
S01B - Antiinflammatory agents
S01BC - Antiinflammatory agents, non-steroids
S01BC01 - Indometacin
Absorption
Indometacin displays a linear pharmacokinetics profile where the plasma concentrations and area under the curve (AUC) are dose-proportional, whereas half-life (T1/2) and plasma and renal clearance are dose-dependent. Indometacin is readily and rapidly absorbed from the gastrointestinal tract. The bioavailability is virtually 100% following oral administration and about 90% of the dose is absorbed within 4 hours. The bioavailability is about 80-90% following rectal administration. The peak plasma concentrations following a single oral dose were achieved between 0.9 0.4 and 1.5 0.8 hours in a fasting state. Despite large intersubject variation as well using the same preparation, peak plasma concentrations are dose-proportional and averaged 1.54 0.76 g/mL, 2.65 1.03 g/mL, and 4.92 1.88 g/mL following 25 mg, 50 mg, and 75 mg single doses in fasting subjects, respectively. With a typical therapeutic regimen of 25 or 50 mg t.i.d., the steady-state plasma concentrations of indomethacin are an average 1.4 times those following the first dose.
Route of Elimination
Indometacin is eliminated via renal excretion, metabolism, and biliary excretion. It is also subject to enter the enterohepatic circulation through excretion of its glucuronide metabolites into bile followed by resorption of indometacin after hydrolysis. The extent of involvement in the enterohepatic circulation ranges from 27 to 115%. About 60 percent of an oral dosage is recovered in urine as drug and metabolites (26 percent as indomethacin and its glucuronide), and 33 percent in the feces (1.5 percent as indomethacin).
Volume of Distribution
The volume of distribution ranged from 0.34 to 1.57 L/kg following oral, intravenous, or rectal administration of single and multiple doses of indometacin in healthy individuals. Indometacin is distributed into the synovial fluid and is extensively bound to tissues. It has been detected in human breast milk and placenta. Although indometacin has been shown to cross the blood-brain barrier (BBB), its extensive plasma protein binding allows only the small fraction of free or unbound indometacin to diffuse across the BBB.
Clearance
In a clinical pharmacokinetic study, the plasma clearance of indometacin was reported to range from 1 to 2.5 mL/kg/min following oral administration.
Patent ductus arteriosus (PDA) is a frequent complication in premature infants. Intravenous indomethacin is the standard mode of medical therapy and has been shown to be efficacious in closing the ductus. In our setup, oral indomethacin is being regularly used for medical treatment of suspected or clinically diagnosed PDA. Non-availability of the parenteral preparation and lack of information regarding the pharmacokinetic disposition of indomethacin in the premature infants in north Indian population led us to conduct this pharmacokinetic study with oral indomethacin. Twenty premature infants with gestational age 30.3 +/- 0.3 wk and birth weight, 1209.8 +/- 39.5 g; admitted to the neonatal unit of the Nehru Hospital, Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh were enrolled in the study. Indomethacin was administered in a single oral dose of 0.2 mg/kg and blood samples were collected through an indwelling vascular catheter at 0 and 1, 2, 4, 8 and 12 hr after administration of indomethacin. Plasma indomethacin concentrations were assayed by spectrofluorometric technique. Large interindividual variability was observed for peak plasma concentrations (Cmax; 137.9 +/- 14.0 ng/mL), elimination half-life (t1/2 el; 21.4 +/- 1.7 hr) and area under the plasma concentrations time curve (AUC0-infinity;4172 +/- 303 ng.hr/mL) in these infants. Variables like birth weight, and sex did not have any sigiificant effect on indomethacin pharmacokinetics. However, the plasma t1/2 el of indomethacin was significantly (P < 0.01) larger in older infants (gestational age > 30 wk) in comparison to younger ones (gestational age < or = 30 wk). There was a negative correlation between gestational age and elimination t1/2 (r = -0.77). In conclusion, indomethacin pharmacokinetics showed a wide variability in premature infants. In view of these findings it can be suggested that infants of smaller gestational age are at greater risk of cumulative toxicity if more than one dose of indomethacin is given. With advancing age, metabolism as well as elimination of drug is faster that may require modification in indomethacin dose to achieve therapeutic response. These preliminary results may be of use in designing future pharmacokinetic studies of oral indomethacin in preterm neonates on a larger sample.
PMID:14604305 Sharma PK et al; Indian J Med Res 117: 164-9 (2003)
Approximately 33% or more of a 25-mg oral dose of indomethacin is excreted in feces principally as demethylated metabolites in their unconjugated forms; 1.5% of fecal drug excretion occurs as indomethacin. Indomethacin and its conjugates undergo enterohepatic circulation.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2087
In one study in healthy fasting adults, peak plasma concentrations of indomethacin occurred in 0.5-2 hours and were about 0.8-2.5 ug/mL following a 25-mg oral dose, and 2.5-4 ug/mL following a 50-mg oral dose. When indomethacin was administered orally to healthy fasting individuals in 25-mg doses 3 times daily, mean steady-state plasma drug concentrations ranged from 0.39-0.63 ug/mL.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2087
In premature neonates, absorption of oral indomethacin appears to be poor and incomplete; bioavailability is reportedly only about 20%. It has been suggested that poor oral absorption of the drug in premature neonates may result from abnormal pH-dependent diffusion and gastric motility and from lower gastric acid secretion. In neonates, gastric emptying time and motility are increased and peristalsis is irregular and unpredictable. In addition, the lack of solubility of the capsule form of indomethacin in aqueous media may present problems in drug delivery and absorption from extemporaneous preparations.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2086
For more Absorption, Distribution and Excretion (Complete) data for INDOMETHACIN (20 total), please visit the HSDB record page.
Indometacin undergoes hepatic metabolism involving glucuronidation, O-desmethylation, and N-deacylation. O-desmethyl-indomethacin, N-deschlorobenzoyl-indomethacin, and O-desmethyl-N-deschlorobenzoyl-indomethacin metabolites and their glucuronides are primarily inactive and have no pharmacological activity. Unconjugated metabolites are also detected in the plasma. Its high bioavailability indicates that indometacin is unlikely to be subject to the first-pass metabolism.
Indomethacin is metabolized in the liver to its glucuronide conjugate and to desmethyl, desbenzoyl, and desmethyl-desbenzoyl metabolites and their glucuronides. These metabolites do not appear to possess anti-inflammatory activity. A portion of the drug is also N-deacylated by a nonmicrosomal system.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2087
Indomethacin has known human metabolites that include (2S,3S,4S,5R)-6-[2-[1-(4-chlorobenzoyl)-5-methoxy-2-methylindol-3-yl]acetyl]oxy-3,4,5-trihydroxyoxane-2-carboxylic acid and O-Desmethylindomethacin.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Indometacin disposition from the plasma is reported to be biphasic, with a half-life of 1 hour during the initial phase and 2.611.2 hours during the second phase. Interindividual and intraindividual variations are possible due to the extensive and sporadic nature of the enterohepatic recycling and biliary discharge of the drug. The mean half-life of oral indomethacin is estimated to be about 4.5 hours. The disposition of intravenous indometacin in preterm neonates was shown to vary across premature infants. In neonates older than 7 days, the mean plasma half-life of intravenous indometacin was approximately 20 hours, ranging from 15 hours in infants weighing more than 1000 g and 21 hours in infants weighing less than 1000 g.
Plasma concentrations of indomethacin have been studied in 5 healthy volunteers after single and multiple doses (25 mg intravenously [iv], 25, 50, and 100 mg orally, 100 mg rectally, and 25 mg three times daily [tid]. In 8 other normal subjects and in 5 patients a 50-mg oral dose of indomethacin was given and the indomethacin concentration was followed from 8 to 32 hr after dosing. ... The half-life of the beta-phase varied between 2.6 and 11.2 hr.
PMID:1100305 Alvan G et al; Clin Pharmacol Ther 18 (3): 364-73 (1975)
In premature neonates, the serum or plasma elimination half-life of indomethacin is inversely related to postnatal age. In a limited number of neonates, the mean plasma half-life of indomethacin has been reported to be about 20-28 hours in those receiving the drug during the first week of life, compared to about 12-19 hours in those receiving the drug after the first week. The elimination half-life in neonates may also be inversely related to body weight. In one study, the plasma indomethacin half-life showed considerable interindividual variation but averaged 21 hours in neonates weighing less than 1 kg and 15 hours in those weighing more than 1 kg. Total body clearance of indomethacin increases with increasing postnatal age. It was suggested that extensive enterohepatic circulation may commonly occur in premature neonates and may contribute to the relatively long half-life of elimination.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2087
In studies in healthy adults or patients with rheumatoid arthritis, the disappearance of indomethacin from plasma appears to be biphasic with a half-life of approximately 1 hour during the initial phase and 2.6-11.2 hours during the second phase; variations in terminal plasma half-life may be due to individual differences in enterohepatic circulation of the drug. There appears to be no difference between plasma half-life in healthy adults and in rheumatoid arthritis patients.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2087
In one study in healthy adults and patients with arthritis, the half-life for disappearance of indomethacin from synovial fluid was 9 hours.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2087
... Twenty premature infants with gestational age 30.3 +/- 0.3 wk and birth weight, 1209.8 +/- 39.5 g; admitted to the neonatal unit of the Nehru Hospital, Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh were enrolled in the study. Indomethacin was administered in a single oral dose of 0.2 mg/kg and blood samples were collected through an indwelling vascular catheter at 0 and 1, 2, 4, 8 and 12 hr after administration of indomethacin. Plasma indomethacin concentrations were assayed by spectrofluorometric technique. Large interindividual variability was observed for peak plasma concentrations (Cmax; 137.9 +/- 14.0 ng/mL), elimination half-life (t1/2 el; 21.4 +/- 1.7 hr) and area under the plasma concentrations time curve (AUC0-infinity;4172 +/- 303 ng.hr/mL) in these infants. Variables like birth weight, and sex did not have any sigiificant effect on indomethacin pharmacokinetics. However, the plasma t1/2 el of indomethacin was significantly (P < 0.01) larger in older infants (gestational age > 30 wk) in comparison to younger ones (gestational age
PMID:14604305 Sharma PK et al; Indian J Med Res 117: 164-9 (2003)
Indometacin is a nonspecific and reversible inhibitor of the cyclo-oxygenase (COX) enzyme or prostaglandin G/H synthase. There are two identified isoforms of COX: COX-1 is universally present in most body tissues and is involved in the synthesis of the prostaglandins and thromboxane A2, while COX-2 is expressed in response to injury or inflammation. Constitutively expressed, the COX-1 enzyme is involved in gastric mucosal protection, platelet, and kidney function by catalyzing the conversion of arachidonic acid to prostaglandin (PG) G2 and PGG2 to PGH2. COX-2 is constitutively expressed and highly inducible by inflammatory stimuli. It is found in the central nervous system, kidneys, uterus, and other organs. COX-2 also catalyzes the conversion of arachidonic acid to PGG2 and PGG2 to PGH2. In the COX-2-mediated pathway, PGH2 is further converted to PGE2 and PGI2 (also known as prostacyclin). PGE2 is involved in mediating inflammation, pain, and fever. Decreasing levels of PGE2 leads to reduced inflammatory reactions. Indometacin is known to inhibit both isoforms of COX, however, with greater selectivity for COX-1, which accounts for its increased adverse gastric effects relative to other NSAIDs. It binds to the enzyme's active site and prevents the interaction between the enzyme and its substrate, arachidonic acid. Indometacin, unlike other NSAIDs, also inhibits phospholipase A2, the enzyme responsible for releasing arachidonic acid from phospholipids. The analgesic, antipyretic and anti-inflammatory effects of indomethacin as well as adverse reactions associated with the drug occur as a result of decreased prostaglandin synthesis. Its antipyretic effects may be due to action on the hypothalamus, resulting in increased peripheral blood flow, vasodilation, and subsequent heat dissipation. The exact mechanism of action of indometacin in inducing closure of a patent ductus arteriosus is not fully understood; however, it is thought to be through inhibition of prostaglandin synthesis. At birth, the ductus arteriosus is normally closed as the tension of the oxygen increases significantly after birth. Patent ductus arteriosus in premature infants is associated with congenital heart malformations where PGE1 mediates an opposite effect to that of oxygen. PGE1 dilates the ductus arteriosus through smooth muscle relaxation and prevents the closure of the ductus arteriosus. By inhibiting the synthesis of prostaglandins, indometacin promotes the closure of ductus arteriosus. Indometacin has been described as possessing anticancer and antiviral properties through activation of protein kinase R (PKR) and downstream phosphorylation of eIF2, inhibiting protein synthesis.
The anti-inflammatory, analgesic, and antipyretic effects of indomethacin and other nonsteroidal anti-inflammatory drugs (NSAIDs), including selective inhibitors of cyclooxygenase-2 (COX-2) (e.g., celecoxib), appear to result from inhibition of prostaglandin synthesis. While the precise mechanism of the anti-inflammatory and analgesic effects of NSAIAs continues to be investigated, these effects appear to be mediated principally through inhibition of the COX-2 isoenzyme at sites of inflammation with subsequent reduction in the synthesis of certain prostaglandins from their arachidonic acid precursors. This effect may be related to inhibition of the synthesis of prostaglandins that are believed to play a role in modulating the rate and extent of leukocyte infiltration during inflammation. Indomethacin also inhibits lysosomal enzyme release from polymorphonuclear leukocytes. Although the mechanism has not been determined, this effect appears to depend on the nature of the stimulus and may not be related to inhibition of prostaglandin synthesis. It has also been postulated that indomethacin, as an inhibitor of phosphodiesterase, may increase intracellular concentrations of cyclic adenosine monophosphate (AMP) which may play a role in the inflammatory response. In supratherapeutic concentrations, indomethacin depresses the synthesis of mucopolysaccharides through uncoupling of oxidative phosphorylation. By inhibiting cyclooxygenase, indomethacin and some other NSAIAs may also interfere with prostaglandin-mediated formation of autoantibodies that are involved in the inflammatory process.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2085
This article reviews the pathogenic mechanism of non-steroidal anti-inflammatory drug (NSAID)-induced gastric damage, focusing on the relation between cyclooxygenase (COX) inhibition and various functional events. NSAIDs, such as indomethacin, at a dose that inhibits prostaglandin (PG) production, enhance gastric motility, resulting in an increase in mucosal permeability, neutrophil infiltration and oxyradical production, and eventually producing gastric lesions. These lesions are prevented by pretreatment with PGE2 and antisecretory drugs, and also via an atropine-sensitive mechanism, not related to antisecretory action. Although neither rofecoxib (a selective COX-2 inhibitor) nor SC-560 (a selective COX-1 inhibitor) alone damages the stomach, the combined administration of these drugs provokes gastric lesions. SC-560, but not rofecoxib, decreases prostaglandin E2 (PGE2) production and causes gastric hypermotility and an increase in mucosal permeability. COX-2 mRNA is expressed in the stomach after administration of indomethacin and SC-560 but not rofecoxib. The up-regulation of indomethacin-induced COX-2 expression is prevented by atropine at a dose that inhibits gastric hypermotility. In addition, selective COX-2 inhibitors have deleterious influences on the stomach when COX-2 is overexpressed under various conditions, including adrenalectomy, arthritis, and Helicobacter pylori-infection. In summary, gastric hypermotility plays a primary role in the pathogenesis of NSAID-induced gastric damage, and the response, causally related with PG deficiency due to COX-1 inhibition, occurs prior to other pathogenic events such as increased mucosal permeability; and the ulcerogenic properties of NSAIDs require the inhibition of both COX-1 and COX-2, the inhibition of COX-1 upregulates COX-2 expression in association with gastric hypermotility, and PGs produced by COX-2 counteract the deleterious effect of COX-1 inhibition.
PMID:22611307 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3351764 Takeuchi K; World J Gastroenterol 18 (18): 2147-60 (2012)
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 4394
Submission : 1982-01-18
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 21984
Submission : 2008-09-16
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 4220
Submission : 1981-03-18
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 5978
Submission : 1985-08-05
Status : Inactive
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2013-02-26
Pay. Date : 2013-02-07
DMF Number : 3136
Submission : 1978-01-23
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 26340
Submission : 2013-04-24
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 2633
Submission : 1976-03-01
Status : Inactive
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2021-08-27
Pay. Date : 2021-07-08
DMF Number : 21740
Submission : 2008-06-28
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 5258
Submission : 1983-07-20
Status : Inactive
Type : II

USDMF
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 8581
Submission : 1990-05-15
Status : Inactive
Type : II

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
43
PharmaCompass offers a list of Indomethacin API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Indomethacin manufacturer or Indomethacin supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Indomethacin manufacturer or Indomethacin supplier.
PharmaCompass also assists you with knowing the Indomethacin API Price utilized in the formulation of products. Indomethacin API Price is not always fixed or binding as the Indomethacin Price is obtained through a variety of data sources. The Indomethacin Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Indomethacin manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Indomethacin, including repackagers and relabelers. The FDA regulates Indomethacin manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Indomethacin API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Indomethacin manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Indomethacin supplier is an individual or a company that provides Indomethacin active pharmaceutical ingredient (API) or Indomethacin finished formulations upon request. The Indomethacin suppliers may include Indomethacin API manufacturers, exporters, distributors and traders.
click here to find a list of Indomethacin suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Indomethacin DMF (Drug Master File) is a document detailing the whole manufacturing process of Indomethacin active pharmaceutical ingredient (API) in detail. Different forms of Indomethacin DMFs exist exist since differing nations have different regulations, such as Indomethacin USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Indomethacin DMF submitted to regulatory agencies in the US is known as a USDMF. Indomethacin USDMF includes data on Indomethacin's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Indomethacin USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Indomethacin suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Indomethacin Drug Master File in Japan (Indomethacin JDMF) empowers Indomethacin API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Indomethacin JDMF during the approval evaluation for pharmaceutical products. At the time of Indomethacin JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Indomethacin suppliers with JDMF on PharmaCompass.
A Indomethacin CEP of the European Pharmacopoeia monograph is often referred to as a Indomethacin Certificate of Suitability (COS). The purpose of a Indomethacin CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Indomethacin EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Indomethacin to their clients by showing that a Indomethacin CEP has been issued for it. The manufacturer submits a Indomethacin CEP (COS) as part of the market authorization procedure, and it takes on the role of a Indomethacin CEP holder for the record. Additionally, the data presented in the Indomethacin CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Indomethacin DMF.
A Indomethacin CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Indomethacin CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Indomethacin suppliers with CEP (COS) on PharmaCompass.
A Indomethacin written confirmation (Indomethacin WC) is an official document issued by a regulatory agency to a Indomethacin manufacturer, verifying that the manufacturing facility of a Indomethacin active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Indomethacin APIs or Indomethacin finished pharmaceutical products to another nation, regulatory agencies frequently require a Indomethacin WC (written confirmation) as part of the regulatory process.
click here to find a list of Indomethacin suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Indomethacin as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Indomethacin API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Indomethacin as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Indomethacin and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Indomethacin NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Indomethacin suppliers with NDC on PharmaCompass.
Indomethacin Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Indomethacin GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Indomethacin GMP manufacturer or Indomethacin GMP API supplier for your needs.
A Indomethacin CoA (Certificate of Analysis) is a formal document that attests to Indomethacin's compliance with Indomethacin specifications and serves as a tool for batch-level quality control.
Indomethacin CoA mostly includes findings from lab analyses of a specific batch. For each Indomethacin CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Indomethacin may be tested according to a variety of international standards, such as European Pharmacopoeia (Indomethacin EP), Indomethacin JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Indomethacin USP).
