
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
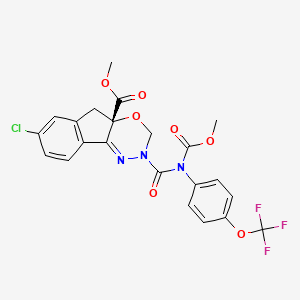

1. Dpx-mp062
1. 173584-44-6
2. (s)-indoxacarb
3. Steward
4. Insecticide
5. Provaunt
6. Advion
7. Avaunt
8. Indoxacarb [iso]
9. 52h0d26mwr
10. Indeno(1,2-e)(1,3,4)oxadiazine-4a(3h)-carboxylic Acid, 7-chloro-2,5-dihydro-2-(((methoxycarbonyl)(4-(trifluoromethoxy)phenyl)amino)carbonyl)-, Methyl Ester, (4as)-
11. Methyl (4as)-7-chloro-2-[methoxycarbonyl-[4-(trifluoromethoxy)phenyl]carbamoyl]-3,5-dihydroindeno[1,2-e][1,3,4]oxadiazine-4a-carboxylate
12. Chebi:38630
13. Indoxacarb (jan)
14. 174060-41-4
15. Methyl (4as)-7-chloro-2-{(methoxycarbonyl)[4-(trifluoromethoxy)phenyl]carbamoyl}-2,5-dihydroindeno[1,2-e][1,3,4]oxadiazine-4a(3h)-carboxylate
16. Avatar
17. Avent
18. Indoxacarb [jan]
19. (+/-)-indoxacarb;dpx-jw 062; Dpx-mp 062; Tornado; Tornado 10fl
20. Dpx-kn 128
21. Hsdb 7280
22. Dpx-mp 062-381
23. Unii-52h0d26mwr
24. Dpx-mp062
25. Indoxacarb 10 Microg/ml In Cyclohexane
26. (4as)-indoxacarb
27. Activyl
28. Indoxacarb [mi]
29. Indoxacarb [hsdb]
30. (+)-indoxacarb
31. Dsstox_cid_12690
32. Dsstox_rid_79034
33. Dsstox_gsid_32690
34. Indoxacarb, (+)-
35. Schembl22073
36. Dpx-kn128
37. Methyl (s)-7chloro-2,5-dihydro-2-(((methoxycarbonyl)(4-(trifluoromethoxy)phenyl)amino)carbonylindeno(1,2-e)(1,3,4)oxadiazine-4a(3h)-carboxylate
38. Chembl197676
39. Dtxsid1032690
40. Amy20519
41. Tox21_300723
42. Zinc28527855
43. Akos037643748
44. Ncgc00164264-02
45. Ncgc00164264-03
46. Ncgc00254629-01
47. As-35129
48. Indoxacarb (ema Epar: Veterinary)
49. Activyl Tick Plus Component Indoxacarb
50. Cas-173584-44-6
51. Indoxacarb, Pestanal(r), Analytical Standard
52. C18569
53. D06316
54. Indoxacarb Component Of Activyl Tick Plus
55. Q421469
56. (s)-methyl 7-chloro-2-(methoxycarbonyl(4-(trifluoromethoxy)phenyl)carbamoyl)-2,3,4a,5-tetrahydroindeno[1,2-e][1,3,4]oxadiazine-4a-carboxylate
57. Indeno[1,2-e][1,3,4]oxadiazine-4a(3h)-carboxylicacid,7-chloro-2,5-dihydro-2-[[(methoxycarbonyl)[4-(trifluoromethoxy)phenyl]amino]carbonyl]-,methyl Ester
58. Methyl (4as)-7-chloro-2,5-dihydro-2-(((methoxycarbonyl)(4-(trifluoromethoxy)phenyl)amino)carbonyl)indeno(1,2-e)(1,3,4)oxadiazine-4a(3h)-carboxylate
59. Pesticide3_indoxacarb_c22h17clf3n3o7_methyl (4as)-7-chloro-2-{(methoxycarbonyl)[4-(trifluoromethoxy)phenyl]carbamoyl}-2,5-dihydroindeno[1,2-e][1,3,4]oxadiazine-4a(3h)-carboxylate
| Molecular Weight | 527.8 g/mol |
|---|---|
| Molecular Formula | C22H17ClF3N3O7 |
| XLogP3 | 4.8 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 5 |
| Exact Mass | 527.0707121 g/mol |
| Monoisotopic Mass | 527.0707121 g/mol |
| Topological Polar Surface Area | 107 Ų |
| Heavy Atom Count | 36 |
| Formal Charge | 0 |
| Complexity | 912 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Treatment and prevention of flea infestation.
For dogs and cats: Treatment and prevention of flea infestation.
The veterinary medicinal product can be used as part of a treatment strategy for flea-allergy dermatitis. Developing stages of fleas in the pet's immediate surroundings are killed following contact with Activyl-treated pets.
QP53AX27
Metabolism in rats after oral dosing was studied using both DPX-JW062 and DPX-MP062. Most of the dose was excreted within 96 hr. Extensive metabolism to numerous minor metabolites occurs. In urine, metabolites were cleaved products (indane or trifluoromethoxyphenyl ring products), whilst in feces, major metabolites retained both these moieties. Major metabolic reactions included hydroxylation of the indane ring, hydrolysis of the carboxymethyl group from the amino nitrogen, and opening of the oxadiazine ring, which gave rise to cleaved products.
Tomlin CDS, ed. Indoxacarb (173584-44-6). In: The e-Pesticide Manual, 13th Edition Version 3.0 (2003-04). Surrey UK, British Crop Protection Council.
... Indoxacarb inhibits sodium channels and certain subtypes of nicotinic receptors.
PMID:12370061 Narahashi T; Mini Rev Med Chem 2 (4): 419-32 (2002)
The effects of the oxadiazine insecticide indoxacarb and its N-decarbomethoxylated metabolite (DCJW) on tetrodotoxin-resistant (TTX-R) voltage-gated sodium channels in rat dorsal ganglion neurons were studied using the whole-cell patch clamp technique. Indoxacarb and DCJW suppressed the peak amplitude of action potentials, and DCJW exhibited a faster time course and higher potency than indoxacarb in the blocking effects. In voltage-clamp experiments, indoxacarb and DCJW suppressed TTX-R sodium currents in a time-dependent manner without a steady-state level of suppression. IC50 values for indoxacarb and DCJW on TTX-R sodium currents were estimated to be 10.7 and 0.8 microM after 25 min of bath application, respectively. DCJW was about 10 times more potent than indoxacarb in blocking TTX-R sodium currents. Although the suppressive effects of indoxacarb were partially reversible after washout with drug-free external solution, no recovery of sodium current was observed in DCJW treated neurons after prolonged washout. In current-voltage relationships, both indoxacarb and DCJW blocked the sodium currents to the same degree in the entire range of membrane potentials. The sodium conductance-voltage curve was not shifted along the voltage axis by indoxacarb and DCJW at 10 microM. In contrast, the steady-state inactivation curves were shifted in the hyperpolarizing direction by indoxacarb as well as by DCJW. Based on these results, it was concluded that indoxacarb and DCJW potently blocked the TTX-R sodium channel in rat DRG neurons with hyperpolarizing shifts of the steady-state inactivation curves, suggesting preferential association of the insecticides to the inactivated state of sodium channels. The small structural variation between indoxacarb and DCJW resulted in clear differences in potency for blocking sodium channels and reversibility after washout.
PMID:12974351 Tsurubuchi Y, Kono Y; Pest Manag Sci 59 (9): 999-1006 (2003)
ABOUT THIS PAGE
65
PharmaCompass offers a list of Indoxacarb API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Indoxacarb manufacturer or Indoxacarb supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Indoxacarb manufacturer or Indoxacarb supplier.
PharmaCompass also assists you with knowing the Indoxacarb API Price utilized in the formulation of products. Indoxacarb API Price is not always fixed or binding as the Indoxacarb Price is obtained through a variety of data sources. The Indoxacarb Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Indoxacarb manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Indoxacarb, including repackagers and relabelers. The FDA regulates Indoxacarb manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Indoxacarb API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Indoxacarb manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Indoxacarb supplier is an individual or a company that provides Indoxacarb active pharmaceutical ingredient (API) or Indoxacarb finished formulations upon request. The Indoxacarb suppliers may include Indoxacarb API manufacturers, exporters, distributors and traders.
click here to find a list of Indoxacarb suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
Indoxacarb Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Indoxacarb GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Indoxacarb GMP manufacturer or Indoxacarb GMP API supplier for your needs.
A Indoxacarb CoA (Certificate of Analysis) is a formal document that attests to Indoxacarb's compliance with Indoxacarb specifications and serves as a tool for batch-level quality control.
Indoxacarb CoA mostly includes findings from lab analyses of a specific batch. For each Indoxacarb CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Indoxacarb may be tested according to a variety of international standards, such as European Pharmacopoeia (Indoxacarb EP), Indoxacarb JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Indoxacarb USP).
