
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Canada

0
Australia

0
South Africa

0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
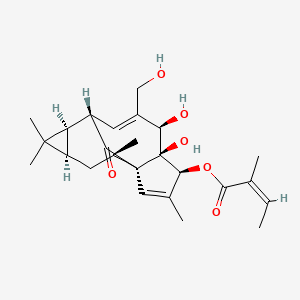

1. 3-ingenol Angelate
2. 3-ingenyl Angelate
3. Ingenol 3-angelate
4. Pep 005
5. Pep-005
6. Pep005
7. Picato
1. Ingenol 3-angelate
2. Picato
3. 3-ingenyl Angelate
4. 3-angeloylingenol
5. Pep-005
6. Pep005
7. 75567-37-2
8. Ingenol-3-angelate
9. Pep 005
10. Ingenol Mebutate [usan]
11. Agn 204332
12. 7686s50jah
13. (1ar,2s,5r,5as,6s,8as,9r,10ar)-5,5a-dihydroxy-4-(hydroxymethyl)-1,1,7,9-tetramethyl-11-oxo-1a,2,5,5a,6,9,10,10a-octahydro-1h-2,8a-methanocyclopenta[a]cyclopropa[e][10]annulen-6-yl (2z)-2-methylbut-2-enoate
14. Ingenol Mebutate (usan)
15. C25h34o6
16. 2-butenoic Acid, 2-methyl-, (1ar,2s,5r,5as,6s,8as,9r,10ar)-1a,2,5,5a,6,9,10,10a-octahydro-5,5a-dihydroxy-4-(hydroxymethyl)-1,1,7,9-tetramethyl-11-oxo-1h-2,8a-methanocyclopenta(a)cyclopropa(e)cyclodecen-6-yl Ester, (2z)-
17. Euphorbia Factor H1
18. Picato (tn)
19. Euphorbia Factor An1
20. Unii-7686s50jah
21. Ingenol Mebutate [usan:inn]
22. Ingenol-mebutate
23. Ingenoli Mebutas
24. [dihydroxy-(hydroxymethyl)-tetramethyl-oxo-[?]yl] (z)-2-methylbut-2-enoate
25. Mebutate D'ingenol
26. Mebutato De Ingenol
27. Ingenol Mebutate [mi]
28. Ingenol Mebutate [inn]
29. Gtpl7443
30. Schembl2526605
31. Chembl1863513
32. Ingenol Mebutate [vandf]
33. Chebi:66913
34. Hsdb 8308
35. Ingenol Mebutate [mart.]
36. Ingenol Mebutate [who-dd]
37. Dtxsid301025610
38. Bdbm50470108
39. Lmfa07010911
40. Mfcd22683801
41. Akos024457952
42. Ingenol Mebutate [orange Book]
43. Zinc100037855
44. Db05013
45. Ingenol-3-angelate, >=95% (hplc)
46. As-79013
47. D09393
48. Q426386
49. (1ar,2s,5r,5as,6s,8as,9r,10ar)-5,5a-dihydroxy-4-(hydroxymethyl)-1,1,7,9-tetramethyl-11-oxo-1a,2,5,5a,6,9,10,10a-octahydro-1h-2,8a- Methanocyclopenta(a)cyclopropa(e)cyclodecen-6-yl (2z)-2-methylbut-2-enoate
50. (1ar,2s,5r,5as,6s,8as,9r,10ar)-5,5a-dihydroxy-4-(hydroxymethyl)-1,1,7,9-tetramethyl-11-oxo-1a,2,5,5a,6,9,10,10a-octahydro-1h-2,8a-methanocyclopenta(a)cyclopropa(e)cyclodecen-6-yl (2z)-2-methylbut-2-enoate
51. (2z)-2-methyl-2-butenoic Acid (1ar,2s,5r,5as,6s,8as,9r,10ar)-1a,2 ,5,5a,6,9,10,10a-octahydro-5,5a-dihydroxy-4-(hydro Xymethyl)-1,1,7,9-tetramethyl-11-oxo-1h-2,8a-metha Nocyclopenta[a]cyclopropa[e]cyclodecen-6-yl Ester
52. (2z)-2-methyl-2-butenoic Acid (1ar,2s,5r,5as,6s,8as,9r,10ar)-1a,2,5,5a,6,9,10,10a-octahydro-5,5a-dihydroxy-4-(hydroxymethyl)-1,1,7,9-tetramethyl-11-oxo-1h-2,8a-methanocyclopenta[a]cyclopropa[e]cyclodecen-6-yl Ester
53. [(1s,4s,5s,6r,9s,10r,12r,14r)-5,6-dihydroxy-7-(hydroxymethyl)-3,11,11,14-tetramethyl-15-oxo-4-tetracyclo[7.5.1.01,5.010,12]pentadeca-2,7-dienyl] (z)-2-methylbut-2-enoate
54. Ingenol Mebutate; (1ar,2s,5r,5as,6s,8as,9r,10ar)-5,5a-dihydroxy-4-(hydroxymethyl)-1,1,7,9-tetramethyl-11-oxo-1a,2,5,5a,6,9,10,10a-octahydro-1h-2,8a-methanocyclopenta[a]cyclopropa[e][10]annulen-6-yl (2z)-2-methylbut-2-enoate
| Molecular Weight | 430.5 g/mol |
|---|---|
| Molecular Formula | C25H34O6 |
| XLogP3 | 2 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 4 |
| Exact Mass | 430.23553880 g/mol |
| Monoisotopic Mass | 430.23553880 g/mol |
| Topological Polar Surface Area | 104 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 926 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 8 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Picato |
| PubMed Health | Ingenol (On the skin) |
| Drug Classes | Dermatological Agent |
| Drug Label | Picato (ingenol mebutate) gel, 0.015% or 0.05% is a clear colorless gel for topical administration, which contains the active substance ingenol mebutate, an inducer of cell death.The chemical name of ingenol mebutate is:2-Butenoic acid, 2-methyl-,... |
| Active Ingredient | Ingenol mebutate |
| Dosage Form | Gel |
| Route | Topical |
| Strength | 0.05%; 0.015% |
| Market Status | Prescription |
| Company | Leo Pharma As |
| 2 of 2 | |
|---|---|
| Drug Name | Picato |
| PubMed Health | Ingenol (On the skin) |
| Drug Classes | Dermatological Agent |
| Drug Label | Picato (ingenol mebutate) gel, 0.015% or 0.05% is a clear colorless gel for topical administration, which contains the active substance ingenol mebutate, an inducer of cell death.The chemical name of ingenol mebutate is:2-Butenoic acid, 2-methyl-,... |
| Active Ingredient | Ingenol mebutate |
| Dosage Form | Gel |
| Route | Topical |
| Strength | 0.05%; 0.015% |
| Market Status | Prescription |
| Company | Leo Pharma As |
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Ingenol mebutate is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of March 17, 2016: https://clinicaltrials.gov/ct2/results?term=Ingenol+mebutate&Search=Search
Picato gel is indicated for the topical treatment of actinic keratosis. /Included in US product label/
NIH; DailyMed. Current Medication Information for Picato (Ingenol Mebutate) Gel (Updated: November 2015). Available from, as of January 20, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5accc7a5-8209-4680-b0ae-2a6963500419
EXPL Although anti-retroviral therapy (ART) is highly effective in suppressing HIV replication, it fails to eradicate the virus from HIV-infected individuals. Stable latent HIV reservoirs are rapidly established early after HIV infection. Therefore, effective strategies for eradication of the HIV reservoirs are urgently needed. We report that ingenol-3-angelate (PEP005), the only active component in a previously FDA approved drug (Picato) for the topical treatment of precancerous actinic keratosis, can effectively reactivate latent HIV in vitro and ex vivo with relatively low cellular toxicity. Biochemical analysis showed that PEP005 reactivated latent HIV through the induction of the pS643/S676-PKCdelta-8-1kappaBalpha/epsilon-NF-kappaB signaling pathway. Importantly, PEP005 alone was sufficient to induce expression of fully elongated and processed HIV RNAs in primary CD4+ T cells from HIV infected individuals receiving suppressive ART. Furthermore, PEP005 and the P-TEFb agonist, JQ1, exhibited synergism in reactivation of latent HIV with a combined effect that is 7.5-fold higher than the effect of PEP005 alone. Conversely, PEP005 suppressed HIV infection of primary CD4+ T cells through down-modulation of cell surface expression of HIV co-receptors. This anti-cancer compound is a potential candidate for advancing HIV eradication strategies.
PMID:2622577 Jiang G et al; PLoS Pathog 11 (7): e1005066 (2015)
EXPL /The purpose of this study was/ to evaluate the safety of two applications of PEP005 (ingenol mebutate) gel in superficial basal cell carcinoma. Efficacy was a secondary end-point. Randomized, vehicle-controlled, phase IIa study conducted at eight private dermatology clinics in Australia /evaluated/ a total of 60 patients with histologically confirmed superficial basal cell carcinoma (lesion size, 4-15 mm). /They/ were randomized to treatment on days 1 and 2 (Arm A) or days 1 and 8 (Arm B) and, within each arm, to ingenol mebutate gel, 0.0025%, 0.01% or 0.05%, or vehicle gel. The main outcome measures were the incidence and severity of adverse events and local skin responses in Arms A and B; lesion clearance at day 85 was a secondary measure. The incidence of adverse events was low. One patient treated with ingenol mebutate gel, 0.05% in Arm A experienced severe flaking/scaling/dryness extending beyond the application site. Non-severe, potentially treatment-related events included erythema extending beyond the application site, application-site pain and headache in two patients each. Six patients in Arm A had one or more severe local skin responses. Efficacy appeared to be dose-related and there was a trend towards higher clinical and histological lesion clearance rates in Arm A compared with Arm B. Histological clearance occurred in five of eight patients (63%) randomized to ingenol mebutate gel, 0.05% in Arm A. Two applications of ingenol mebutate gel, 0.05%, are safe and have efficacy in patients with superficial basal cell carcinoma.
PMID:20546215 Siller G et al; Australas J Dermatol 51 (2): 99-105 (2010)
Hypersensitivity reactions, including anaphylaxis and allergic contact dermatitis, have been reported post-marketing. If anaphylactic or other clinically significant hypersensitivity reactions occur, discontinue Picato gel immediately and institute appropriate medical therapy.
NIH; DailyMed. Current Medication Information for Picato (Ingenol Mebutate) Gel (Updated: November 2015). Available from, as of January 20, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5accc7a5-8209-4680-b0ae-2a6963500419
Avoid treatment in the periocular area. Eye disorders, including severe eye pain, chemical conjunctivitis, corneal burn, eyelid edema, eyelid ptosis, periorbital edema can occur after exposure.
NIH; DailyMed. Current Medication Information for Picato (Ingenol Mebutate) Gel (Updated: November 2015). Available from, as of January 20, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5accc7a5-8209-4680-b0ae-2a6963500419
The following adverse reactions have been identified during post approval use of Picato (ingenol mebutate) gel, 0.015% and 0.05%: hypersensitivity, allergic contact dermatitis, herpes zoster, chemical conjunctivitis, and corneal burn.
NIH; DailyMed. Current Medication Information for Picato (Ingenol Mebutate) Gel (Updated: November 2015). Available from, as of January 20, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5accc7a5-8209-4680-b0ae-2a6963500419
Severe skin reactions in the treated area, including erythema, crusting, swelling, vesiculation/postulation, and erosion/ulceration, can occur after topical application of Picato gel. Administration of Picato gel is not recommended until the skin is healed from any previous drug or surgical treatment.
NIH; DailyMed. Current Medication Information for Picato (Ingenol Mebutate) Gel (Updated: November 2015). Available from, as of January 20, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5accc7a5-8209-4680-b0ae-2a6963500419
For more Drug Warnings (Complete) data for Ingenol mebutate (12 total), please visit the HSDB record page.
For the topical treatment of actinic keratosis.
FDA Label
Picato is indicated for the cutaneous treatment of nonhyperkeratotic, nonhypertrophic actinic keratosis in adults.
The pharmacodynamics of ingenol mebutate in producing cell death in actinic keratosis is unknown.
D06BX02
D - Dermatologicals
D06 - Antibiotics and chemotherapeutics for dermatological use
D06B - Chemotherapeutics for topical use
D06BX - Other chemotherapeutics
D06BX02 - Ingenol mebutate
Absorption
Since ingenol mebutate is a topical treatment, the systemic absorption is less than 0.1 ng/mL.
Route of Elimination
There is no route of elimination since ingenol mebutate is a topical treatment.
Volume of Distribution
There is no volume of distribution quantity since ingenol mebutate is a topical treatment.
Clearance
There is no clearance quantity since ingenol mebutate is a topical treatment.
Plasma clearance and volume of distribution (steady-state) in humans were estimated using a simple allometric correlation based on body weight. Using a one-compartment model with first-order absorption and elimination kinetics, it was estimated that dermal administration of the maximum intended clinical dose of 2 ug/kg/day would produce levels of ingenol mebutate in the blood below the LLOQ of 0.1 ng/mL. Blood clearance and volume of distribution at steady-state were predicted to range from approximately 0.22 to 1.01 L/hr/kg and approximately 0.61 L/kg, respectively. The absorption rate constant and topical bioavailability was projected to be 0.0277 hours-1 and 0.21%, respectively. A human blood Tmax of 2 hours and Cmax of 0.107 pg/mL were predicted for a 2 ug/kg/day topical dose. A minimum topical dose of 2000 ug/kg/day to humans would be required produce detectable blood levels.
European Medicines Agency (EMA), Committee for Medicinal Products for Human Use (CHMP), European Public Assessment Report (EPAR): Picato (Ingenol Mebutate) p.25 (2012). Available from, as of January 28, 2016: https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002275/WC500135329.pdf
After IV administration, a high to very high blood clearance, moderate to high volume of distribution at steady-state and short half-life were observed in rats, rabbits, dogs and minipigs. Following IV administration of 3(H)-ingenol mebutate to rats, drug-related radioactivity was well distributed to the tissues and there were no gender differences in the organs exposed but elimination was faster in females. In vitro, ingenol mebutate and its isomers were shown to have high plasma protein binding in rats, dogs, minipigs and humans (>99%). In rats, the majority of an intravenous dose of 3(H)-ingenol mebutate was excreted via the biliary route, with urinary excretion as a minor pathway.
European Medicines Agency (EMA), Committee for Medicinal Products for Human Use (CHMP), European Public Assessment Report (EPAR): Picato (Ingenol Mebutate) p.24 (2012). Available from, as of January 28, 2016: https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002275/WC500135329.pdf
After in vitro applications of 0.01%, 0.1% or 0.05% PEP005 Gels to rat, mini-pig and human skin preparations, the percutaneous absorption was generally low, with a range of 0.04% (mini-pig) to 8.68% (rat) across animal species and 0.16% to 1.93% in humans. The absorbed doses of 3(H)-ingenol mebutate were in the order of WI rat > SD rat > human > mini-pig. After topical administration of PEP005 Gel to mini-pigs, blood levels of ingenol mebutate were generally not detected, and when detected, ranged up to 0.1 ng/mL. After topical administration of ingenol mebutate to rats, blood levels were consistently quantifiable only at doses of 300 ug/kg or greater, in which case the absolute bioavailability was 2% to 4%.
European Medicines Agency (EMA), Committee for Medicinal Products for Human Use (CHMP), European Public Assessment Report (EPAR): Picato (Ingenol Mebutate) p.24 (2012). Available from, as of January 28, 2016: https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002275/WC500135329.pdf
The systemic exposure to Picato gel, 0.05% was assessed in two studies in a total of 16 subjects with AK, following application of approximately 1 g of Picato gel, 0.05% to an area of 100 cm2 of the dorsal forearm once daily for two consecutive days. In these studies, the blood levels of ingenol mebutate and two of its metabolites (acyl isomers of ingenol mebutate) were measured. Blood levels of ingenol mebutate and the two metabolites were below the lower limit of quantification (0.1 ng/mL) in all the blood samples of the subjects evaluated.
NIH; DailyMed. Current Medication Information for Picato (Ingenol Mebutate) Gel (Updated: November 2015). Available from, as of January 28, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5accc7a5-8209-4680-b0ae-2a6963500419
There is no metabolism of Picato since ingenol mebutate is a topical treatment, and ingenol mebutate does not inhibit or induce a majority of the cytochrome P450 (CYP) enzymes.
The in vitro metabolism of ingenol mebutate was qualitatively similar in blood, skin homogenates and hepatocytes of rats, dogs, minipigs and humans. Ingenol mebutate was found to be relatively stable in blood and skin homogenates, and to undergo extensive metabolism in cryopreserved hepatocytes. The major pathway in rat, dog and minipig hepatocytes was hydrolysis to ingenol, whereas the major pathway in humans was hydroxylation of ingenol mebutate. In the skin of rats, dogs, minipigs and humans, rearrangement of ingenol mebutate was predominantly to PEP015 (approximately 26% to approximately 31%) and, to a much lesser extent, PEP025 (approximately 1% to approximately 2%); hydrolysis to ingenol was minimal (0% to 0.81%). However, after topical or IV administration of ingenol mebutate to rats and minipigs, PEP025 was not detected and PEP015 was less than 10% of the corresponding ingenol mebutate concentration in the blood.
European Medicines Agency (EMA), Committee for Medicinal Products for Human Use (CHMP), European Public Assessment Report (EPAR): Picato (Ingenol Mebutate) p.25 (2012). Available from, as of January 28, 2016: https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002275/WC500135329.pdf
There is no half-life quantity since ingenol mebutate is a topical treatment.
The exact mechanism of action of ingenol mebutate in actinic keratosis is unknown. It is presumed to involve primary necrosis then neutrophil-mediated inflammation and antibody-dependent cell death of residual disease cells. Additionally in early studies, PEP005 was shown to be an effective activator of PKC-delta and PKC-delta translocation into nucleus and membranes. PEP005 also downregulates the expression and activity of PKC-alpha. PEP005 induced modulation of PKCs leads to Ras/Raf/MAPK and p38 activation and AKT/PKB inhibition.
We investigated the proposed necrotic mechanism of ingenol mebutate, a natural compound with anti-cancer properties in human keratinocytes, the human squamous cell carcinoma cell line HSC-5, and HeLa cervix carcinoma cells. Topical application of a clinical dose of ingenol mebutate 0.05% (1.15 mM) gel to human reconstituted full-thickness skin equivalents strongly reduced epidermal, but not dermal viability. Ingenol mebutate showed cytotoxic potency between 200-300 uM on normal and cancer cells. When keratinocytes were induced to differentiate, they became significantly less sensitive to ingenol mebutate and half-maximal induction of cell death required more than 300 uM ingenol mebutate. Cytotoxic concentrations of ingenol mebutate caused rupture of the mitochondrial network within minutes paralleled by cytosolic calcium release in all cells. Subsequently, plasma membrane integrity was lost as seen by propidium uptake into the cells. This was in sharp contrast to lysis of cells with low concentrations of the detergent Triton X-100 that permeabilized the plasma membrane within minutes without affecting organelle morphology. Buffering of intracellular calcium and inhibition of the mitochondrial permeability transition pore reduced the cytotoxic effect of ingenol mebutate in cancer cells, but not in normal keratinocytes. However, these inhibitors could not prevent cell death subsequent to prolonged incubation. Our findings reveal that ingenol mebutate does not mediate cytotoxicity by a simple lytic, necrotic mechanism, but activates distinct processes involving multiple cell organelles in a cell-type and differentiation-dependent manner. These data improve our understanding of ingenol mebutate-target cell interactions and offer new insights relevant to the removal of aberrant cells in human skin.
PMID:23134983 Stahlhut M et al; J Drugs Dermatol 11 (10): 1181-92 (2012)
Squamous cell carcinoma (SCC) is the second most common human skin cancer and the second leading cause of skin cancer-related death. Recently, a new compound, ingenol mebutate, was approved for treatment of actinic keratosis, a precursor of SCC. As the mechanism of action is poorly understood, we have further investigated the mechanism of ingenol mebutate-induced cell death. We elucidate direct effects of ingenol mebutate on primary keratinocytes, patient-derived SCC cells, and a SCC cell line. Transcriptional profiling followed by pathway analysis was performed on ingenol mebutate-treated primary keratinocytes and patient-derived SCC cells to find key mediators and identify the mechanism of action. Activation of the resulting pathways was confirmed in cells and human skin explants and supported by a phosphorylation screen of treated primary cells. The necessity of these pathways was demonstrated by inhibition of certain pathway components. Ingenol mebutate inhibited viability and proliferation of all keratinocyte-derived cells in a biphasic manner. Transcriptional profiling identified the involvement of PKC/MEK/ERK signaling in the mechanism of action and inhibition of this signaling pathway rescued ingenol mebutate-induced cell death after treatment with 100 nmol/L ingenol mebutate, the optimal concentration for the first peak of response. We found the interleukin decoy receptors IL1R2 and IL13RA2 induced by ingenol mebutate in a PKC/MEK/ERK-dependent manner. Furthermore, siRNA knockdown of IL1R2 and IL13RA2 partially rescued ingenol mebutate-treated cells. In conclusion, we have shown that ingenol mebutate-induced cell death is mediated through the PKCd/MEK/ERK pathway, and we have functionally linked the downstream induction of IL1R2 and IL13RA2 expression to the reduced viability of ingenol mebutate-treated cells.
PMID:26116359 Freiberger SN et al; Mol Cancer Ther. 2015 Sep;14(9):2132-42 (2015)
Members of the protein kinase C (PKC) family of serine-threonine kinases are important regulators of immune cell survival. Ingenol 3-angelate (PEP005) activates a broad range of PKC isoforms and induces apoptosis in acute myeloid leukemia cells by activating the PKC isoform PKCdelta. We show here that, in contrast to its effect on leukemic cells, PEP005 provides a strong survival signal to resting and activated human T cells. The antiapoptotic effect depends upon the activation of PKC. This PKC isoform is expressed in T cells but is absent in myeloid cells. Further studies of the mechanism involved in this process showed that PEP005 inhibited activated CD8(+) T cell apoptosis through the activation of NFkappaB downstream of PKC, leading to increased expression of the antiapoptotic proteins Mcl-1 and Bcl-x(L). Transfection of CD8(+) T cells with dominant-negative PKC diminished the prosurvival effect of PEP005 significantly. Ectopic expression of PKC in the acute myeloid leukemia cell line NB4 turned their response to PEP005 from an increased to decreased rate of apoptosis. Therefore, in contrast to myeloid leukemia cells, PEP005 provides a strong survival signal to T cells, and the expression of functional PKC influences whether PKC activation leads to an anti- or proapoptotic outcome in the cell types tested.
PMID:20472553 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2911273 Lee WY et al; J Biol Chem 285 (31): 23889-98 (2010)
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
32
PharmaCompass offers a list of Ingenol Mebutate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Ingenol Mebutate manufacturer or Ingenol Mebutate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Ingenol Mebutate manufacturer or Ingenol Mebutate supplier.
PharmaCompass also assists you with knowing the Ingenol Mebutate API Price utilized in the formulation of products. Ingenol Mebutate API Price is not always fixed or binding as the Ingenol Mebutate Price is obtained through a variety of data sources. The Ingenol Mebutate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Ingenol Mebutate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Ingenol Mebutate, including repackagers and relabelers. The FDA regulates Ingenol Mebutate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Ingenol Mebutate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Ingenol Mebutate manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Ingenol Mebutate supplier is an individual or a company that provides Ingenol Mebutate active pharmaceutical ingredient (API) or Ingenol Mebutate finished formulations upon request. The Ingenol Mebutate suppliers may include Ingenol Mebutate API manufacturers, exporters, distributors and traders.
click here to find a list of Ingenol Mebutate suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Ingenol Mebutate DMF (Drug Master File) is a document detailing the whole manufacturing process of Ingenol Mebutate active pharmaceutical ingredient (API) in detail. Different forms of Ingenol Mebutate DMFs exist exist since differing nations have different regulations, such as Ingenol Mebutate USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Ingenol Mebutate DMF submitted to regulatory agencies in the US is known as a USDMF. Ingenol Mebutate USDMF includes data on Ingenol Mebutate's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Ingenol Mebutate USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Ingenol Mebutate suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Ingenol Mebutate Drug Master File in Korea (Ingenol Mebutate KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Ingenol Mebutate. The MFDS reviews the Ingenol Mebutate KDMF as part of the drug registration process and uses the information provided in the Ingenol Mebutate KDMF to evaluate the safety and efficacy of the drug.
After submitting a Ingenol Mebutate KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Ingenol Mebutate API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Ingenol Mebutate suppliers with KDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Ingenol Mebutate as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Ingenol Mebutate API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Ingenol Mebutate as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Ingenol Mebutate and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Ingenol Mebutate NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Ingenol Mebutate suppliers with NDC on PharmaCompass.
Ingenol Mebutate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Ingenol Mebutate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Ingenol Mebutate GMP manufacturer or Ingenol Mebutate GMP API supplier for your needs.
A Ingenol Mebutate CoA (Certificate of Analysis) is a formal document that attests to Ingenol Mebutate's compliance with Ingenol Mebutate specifications and serves as a tool for batch-level quality control.
Ingenol Mebutate CoA mostly includes findings from lab analyses of a specific batch. For each Ingenol Mebutate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Ingenol Mebutate may be tested according to a variety of international standards, such as European Pharmacopoeia (Ingenol Mebutate EP), Ingenol Mebutate JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Ingenol Mebutate USP).
