
Synopsis
Synopsis
0
CEP/COS
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Canada

0
Australia

DRUG PRODUCT COMPOSITIONS
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Ifn-alpha 2
2. Ifn-alpha-2
3. Ifnalpha 2b, Recombinant
4. Ifnalpha-2b, Recombinant
5. Interferon Alfa 2a
6. Interferon Alfa 2b
7. Interferon Alfa-2a
8. Interferon Alpha 2
9. Interferon Alpha 2b, Recombinant
10. Interferon Alpha A
11. Interferon Alpha-2
12. Interferon Alpha-2a, Recombinant
13. Interferon Alpha-2b, Recombinant
14. Interferon Alpha-a
15. Interferon-alpha 2
16. Intron A (interferon)
17. Leif A
18. Reaferon
19. Recombinant Ifnalpha-2b
20. Recombinant Interferon Alpha 2a
21. Recombinant Interferon Alpha 2b
22. Recombinant Interferon Alpha-2a
23. Recombinant Interferon Alpha-2b
24. Ro 22 8181
25. Ro 22-8181
26. Ro 228181
27. Roferon A
28. Roferon-a
29. Roferona
30. Sch 30500
31. Sch-30500
32. Sch30500
33. Viferon
1. Interferon Alfa-2b, Recombinant
2. 98530-12-2
3. Schembl6041041
4. Q4391540
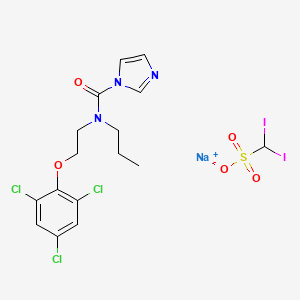
| Molecular Weight | 746.5 g/mol |
|---|---|
| Molecular Formula | C16H17Cl3I2N3NaO5S |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 7 |
| Exact Mass | 744.79416 g/mol |
| Monoisotopic Mass | 744.79416 g/mol |
| Topological Polar Surface Area | 113 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 511 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
Recombinant Proteins; Interferon-alpha
National Library of Medicine's Medical Subject Headings online file (MeSH, 2013)
INTRON A is indicated for the treatment of patients 18 years of age or older with hairy cell leukemia. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for INTRON A (interferon alfa-2b) kit (August 2012). Available from, as of August 2, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=fd653d74-48ab-49e4-a42d-ec1cbc59badb
INTRON A is indicated for the treatment of selected patients 18 years of age or older with AIDS-Related Kaposi's Sarcoma. The likelihood of response to INTRON A therapy is greater in patients who are without systemic symptoms, who have limited lymphadenopathy and who have a relatively intact immune system as indicated by total CD4 count. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for INTRON A (interferon alfa-2b) kit (August 2012). Available from, as of August 2, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=fd653d74-48ab-49e4-a42d-ec1cbc59badb
INTRON A is indicated for the initial treatment of clinically aggressive follicular Non-Hodgkin's Lymphoma in conjunction with anthracycline-containing combination chemotherapy in patients 18 years of age or older. Efficacy of INTRON A therapy in patients with low-grade, low-tumor burden follicular Non-Hodgkin's Lymphoma has not been demonstrated. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for INTRON A (interferon alfa-2b) kit (August 2012). Available from, as of August 2, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=fd653d74-48ab-49e4-a42d-ec1cbc59badb
For more Therapeutic Uses (Complete) data for Interferon Alfa-2b (17 total), please visit the HSDB record page.
/BOXED WARNING/ Alpha interferons, including INTRON A, cause or aggravate fatal or life-threatening neuropsychiatric, autoimmune, ischemic, and infectious disorders. Patients should be monitored closely with periodic clinical and laboratory evaluations. Patients with persistently severe or worsening signs or symptoms of these conditions should be withdrawn from therapy. In many but not all cases these disorders resolve after stopping INTRON A therapy.
US Natl Inst Health; DailyMed. Current Medication Information for INTRON A (interferon alfa-2b) kit (August 2012). Available from, as of August 2, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=fd653d74-48ab-49e4-a42d-ec1cbc59badb
Almost all patients experience adverse effects at some time during the course of interferon alfa therapy. However, evaluation of some adverse effects and establishment of a causal relationship to interferon alfa have been difficult since the drug has been used principally in patients with serious underlying diseases, such as acquired immunodeficiency syndrome (AIDS), various cancers, and/or viral hepatitis.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 1072
A flu-like syndrome develops to some degree in almost all patients receiving 1 million units or more of interferon alfa but its severity appears to be dose related. The syndrome is characterized by the development of fever (in about 40-98% of patients), fatigue/malaise (in about 50-95% of patients), myalgia (in about 30-75% of patients), chills (in about 40-65% of patients), headache (in about 20-70% of patients), arthralgia (in about 5-24% of patients), rigors, tachycardia, anorexia, dry mouth, dysgeusia, back pain, sweating, and dizziness. Abdominal cramps and diarrhea also may be associated with the syndrome.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 1072
Myalgia and arthralgia, often associated with a flu-like syndrome, are the most frequent adverse musculoskeletal effects of interferon alfa, occurring in up to 70 and 24% of patients, respectively. These effects generally are transient, mild, and self-limiting. More severe myalgias, generally involving the lower extremities and associated with limitation of movement, have been observed in patients with chronic myelogenous leukemia receiving the drug. Such myalgias frequently require 1-2 weeks of bed rest and corticosteroid and/or analgesic (e.g., opiate) therapy for relief. These severe myalgias generally were not associated with an increase in serum muscle enzymes, and electromyograms have failed to reveal evidence of myositis. Skeletal pain, which frequently is one of the first adverse effects associated with interferon alfa therapy, has been reported in some patients with multiple myeloma who were receiving the drug; however, arthralgias were not observed in patients with metastatic osseous lesions associated with renal cell carcinoma or other malignancies.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 1073
For more Drug Warnings (Complete) data for Interferon Alfa-2b (47 total), please visit the HSDB record page.
For the treatment of hairy cell leukemia, malignant melanoma, and AIDS-related Kaposi's sarcoma.
FDA Label
* Chronic hepatitis B:
Treatment of adult patients with chronic hepatitis B associated with evidence of hepatitis-B viral replication (presence of DNA of hepatitis-B virus (HBV-DNA) and hepatitis-B antigen (HBeAg), elevated alanine aminotransferase (ALT) and histologically proven active liver inflammation and / or fibrosis.
* Chronic hepatitis C:
Before initiating treatment with IntronA, consideration should be given to the results from clinical trials comparing IntronA with pegylated interferon.
Adult patients
IntronA is indicated for the treatment of adult patients with chronic hepatitis C who have elevated transaminases without liver decompensation and who are positive for hepatitis-C virus-RNA (HCV-RNA).
The best way to use IntronA in this indication is in combination with ribavirin.
Children three years of age and older and adolescents
IntronA is indicated, in a combination regimen with ribavirin, for the treatment of children three years of age and older and adolescents, who have chronic hepatitis C, not previously treated, without liver decompensation, and who are positive for HCV-RNA. When deciding not to defer treatment until adulthood, it is important to consider that the combination therapy induced a growth inhibition that resulted in reduced final adult height in some patients.
The decision to treat should be made on a case-by-case basis.
* Hairy-cell leukaemia:
Treatment of patients with hairy cell leukaemia.
* Chronic myelogenous leukaemia:
Monotherapy
Treatment of adult patients with Philadelphia-chromosome- or bcr/abl-translocation-positive chronic myelogenous leukaemia.
Clinical experience indicates that a haematological and cytogenetic major / minor response is obtainable in the majority of patients treated. A major cytogenetic response is defined by < 34 % Ph+ leukaemic cells in the bone marrow, whereas a minor response is 34 %, but < 90 % Ph+ cells in the marrow.
Combination therapy
The combination of interferon alfa-2b and cytarabine (Ara-C) administered during the first 12 months of treatment has been demonstrated to significantly increase the rate of major cytogenetic responses and to significantly prolong the overall survival at three years when compared to interferon alfa-2b monotherapy.
* Multiple myeloma:
As maintenance therapy in patients who have achieved objective remission (more than 50% reduction in myeloma protein) following initial induction chemotherapy.
Current clinical experience indicates that maintenance therapy with interferon alfa-2b prolongs the plateau phase; however, effects on overall survival have not been conclusively demonstrated.
* Follicular lymphoma:
Treatment of high-tumour-burden follicular lymphoma as adjunct to appropriate combination induction chemotherapy such as a CHOP-like regimen. High tumour burden is defined as having at least one of the following: bulky tumour mass (> 7 cm), involvement of three or more nodal sites (each > 3 cm), systemic symptoms (weight loss > 10 %, pyrexia > 38C for more than eight days, or nocturnal sweats), splenomegaly beyond the umbilicus, major organ obstruction or compression syndrome, orbital or epidural involvement, serous effusion, or leukaemia.
* Carcinoid tumour:
Treatment of carcinoid tumours with lymph node or liver metastases and with 'carcinoid syndrome'.
* Malignant melanoma:
As adjuvant therapy in patients who are free of disease after surgery but are at high risk of systemic recurrence, e. g. patients with primary or recurrent (clinical or pathological) lymph-node.
* Chronic Hepatitis B: :
Treatment of adult patients with chronic hepatitis B associated with evidence of hepatitis B viral replication (presence of HBV-DNA and HBeAg), elevated alanine aminotransferase (ALT) and histologically proven active liver inflammation and/or fibrosis.
* Chronic Hepatitis C: :
Adult patients:
IntronA is indicated for the treatment of adult patients with chronic hepatitis C who have elevated transaminases without liver decompensation and who are positive for serum HCV-RNA or anti-HCV (see section 4. 4).
The best way to use IntronA in this indication is in combination with ribavirin.
Chidren and adolescents:
IntronA is intended for use, in a combination regimen with ribavirin, for the treatment of children and adolescents 3 years of age and older, who have chronic hepatitis C, not previously treated, without liver decompensation, and who are positive for serum HCV-RNA. The decision to treat should be made on a case by case basis, taking into account any evidence of disease progression such as hepatic inflammation and fibrosis, as well as prognostic factors for response, HCV genotype and viral load. The expected benefit of treatment should be weighed against the safety findings observed for paediatric subjects in the clinical trials (see sections 4. 4, 4. 8 and 5. 1).
Upregulates the expression of MHC I proteins, allowing for increased presentation of peptides derived from viral antigens. This enhances the activation of CD8+ T cells that are the precursors for cytotoxic T lymphocytes (CTLs) and makes the macrophage a better target for CTL-mediated killing. Interferon alpha also induce the synthesis of several key antiviral mediators, including 2'-5' oligoadenylate synthetase (2'-5' A synthetase) and protein kinase R.
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
Antiviral Agents
Agents used in the prophylaxis or therapy of VIRUS DISEASES. Some of the ways they may act include preventing viral replication by inhibiting viral DNA polymerase; binding to specific cell-surface receptors and inhibiting viral penetration or uncoating; inhibiting viral protein synthesis; or blocking late stages of virus assembly. (See all compounds classified as Antiviral Agents.)
L03AB05
L03AB05
L - Antineoplastic and immunomodulating agents
L03 - Immunostimulants
L03A - Immunostimulants
L03AB - Interferons
L03AB05 - Interferon alfa-2b
Absorption
Absorption is high (greater than 80%) when administered intramuscularly or subcutaneously.
For systemic effects, interferon alfa is administered parenterally because the drug is susceptible to degradation by proteolytic enzymes in the GI tract. Interferon alfa is well absorbed following IM or subcutaneous injection; the apparent fraction of the dose absorbed after IM or subcutaneous injection exceeds 80%. Peak serum interferon alfa concentrations following IV administration of the drug generally occur within 15-60 minutes and are substantially greater than those attained after IM or subcutaneous administration. However, serum interferon alfa concentrations following IM or subcutaneous administration generally are maintained for longer periods of time than those produced by rapid IV injection or rapid (e.g., 40 minutes or less) IV infusion.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 1084
After intravenous administration, serum INTRON A concentrations peaked (135-273 IU/mL) by the end of the 30-minute infusion, then declined at a slightly more rapid rate than after intramuscular or subcutaneous drug administration, becoming undetectable 4 hours after the infusion.
US Natl Inst Health; DailyMed. Current Medication Information for INTRON A (interferon alfa-2b) kit (August 2012). Available from, as of August 2, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=fd653d74-48ab-49e4-a42d-ec1cbc59badb
The pharmacokinetics of INTRON A were studied in 12 healthy male volunteers following single doses of 5 million IU/sq m administered intramuscularly, subcutaneously, and as a 30-minute intravenous infusion in a crossover design. The mean serum INTRON A concentrations following intramuscular and subcutaneous injections were comparable. The maximum serum concentrations obtained via these routes were approximately 18 to 116 IU/mL and occurred 3 to 12 hours after administration. Serum concentrations were undetectable by 16 hours after the injections.
US Natl Inst Health; DailyMed. Current Medication Information for INTRON A (interferon alfa-2b) kit (August 2012). Available from, as of August 2, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=fd653d74-48ab-49e4-a42d-ec1cbc59badb
Limited data on the tissue distribution of interferon in animals suggest that mixtures of naturally occurring human or animal interferons are widely and rapidly distributed into body tissues after parenteral administration, with the highest concentrations occurring in spleen, kidney, liver, and lung.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 1084
For more Absorption, Distribution and Excretion (Complete) data for Interferon Alfa-2b (7 total), please visit the HSDB record page.
The elimination half-life following both intramuscular and subcutaneous injections was approximately 2 to 3 hours. The elimination half-life was approximately 2 hours following intravenous injection.
The elimination half-life was approximately 2 hours /after intravenous administration/.
US Natl Inst Health; DailyMed. Current Medication Information for INTRON A (interferon alfa-2b) kit (August 2012). Available from, as of August 2, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=fd653d74-48ab-49e4-a42d-ec1cbc59badb
The elimination half-life of INTRON A following both intramuscular and subcutaneous injections was approximately 2 to 3 hours.
US Natl Inst Health; DailyMed. Current Medication Information for INTRON A (interferon alfa-2b) kit (August 2012). Available from, as of August 2, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=fd653d74-48ab-49e4-a42d-ec1cbc59badb
Interferon alpha binds to type I interferon receptors (IFNAR1 and IFNAR2c) which upon dimerization activate two Jak (Janus kinase) tyrosine kinases (Jak1 and Tyk2). These transphosphorylate themselves and phosphorylate the receptors. The phosphorylated INFAR receptors then bind to Stat1 and Stat2 (signal transducers and activators of transcription)which dimerize and activate multiple (~100) immunomodulatory and antiviral proteins. Interferon alpha binds less stably to type I interferon receptors than interferon beta.
Interferons exert their cellular activities by binding to specific membrane receptors on the cell surface. Once bound to the cell membrane, interferons initiate a complex sequence of intracellular events. In vitro studies demonstrated that these include the induction of certain enzymes, suppression of cell proliferation, immunomodulating activities such as enhancement of the phagocytic activity of macrophages and augmentation of the specific cytotoxicity of lymphocytes for target cells, and inhibition of virus replication in virus-infected cells.
US Natl Inst Health; DailyMed. Current Medication Information for INTRON A (interferon alfa-2b) kit (August 2012). Available from, as of August 2, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=fd653d74-48ab-49e4-a42d-ec1cbc59badb
Following binding to specific cellular receptors, interferons (INFs) activate the JAK_STAT signal-transduction pathway and lead to the nuclear translocation of a cellular protein complex that binds to genes containing an INF-specific response element. This, in turn, leads to the synthesis of over two dozen proteins that contribute to viral resistance mediated at different stages of viral penetration. Inhibition of protein synthesis is the major inhibitory effect for many viruses. INF-induced proteins include 2',5'-oligoadenylate (2-5(A)) synthetase and a protein kinase, either of which can inhibit protein synthesis in the presence of double stranded RNA. The 2-5(A) synthetase produces adenylate oligomers that activate a latent cellular endoribonuclease (RNase L) to cleave both cellular and viral single stranded RNAs. The protein kinase selectively phosphorylates and inactivates a protein involved in protein synthesis, eukaryotic initiation factor 2 (eIF-2). IFN-induced protein kinase also may be an important effector of apoptosis. In addition, INF induces a phosphodiesterase that cleaves a portion of transfer RNA and thus prevents peptide elongation.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 11th ed. New York, NY: McGraw-Hill, 2006., p. 1261
The precise mechanisms of action of interferons have not been fully elucidated but appear to be complex, and the resultant activities appear to be substantially interrelated. Unlike classic antiviral and cytotoxic agents, the antiviral and antineoplastic properties of interferons appear to result from a complex cascade of biologic modulation and pharmacologic effects rather than from direct virucidal or cytocidal effects. The drugs affect many cell functions producing restoration, augmentation, and/or modulation of the host's immune system; direct antiproliferative and antineoplastic activities; modulation of cell differentiation; and modulation of cellular transcription and translation, including a reduction in oncogene expression. Some or all of these effects may be interrelated and ultimately responsible for the antiviral and antineoplastic activity of interferons. Interferons must bind to specific cell surface receptors in order to exert biologic and pharmacologic effects (e.g., antiviral activity); such binding appears to involve high-affinity sites. In addition, some evidence suggests that the principal effects of interferons result not from direct intracellular actions but rather from ligand-receptor complexes at the cell surface that can mediate and induce intracellular events.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 1080
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
73
PharmaCompass offers a list of Interferon Alfa-2B API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Interferon Alfa-2B manufacturer or Interferon Alfa-2B supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Interferon Alfa-2B manufacturer or Interferon Alfa-2B supplier.
PharmaCompass also assists you with knowing the Interferon Alfa-2B API Price utilized in the formulation of products. Interferon Alfa-2B API Price is not always fixed or binding as the Interferon Alfa-2B Price is obtained through a variety of data sources. The Interferon Alfa-2B Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Interferon Alfa 2b manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Interferon Alfa 2b, including repackagers and relabelers. The FDA regulates Interferon Alfa 2b manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Interferon Alfa 2b API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Interferon Alfa 2b manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Interferon Alfa 2b supplier is an individual or a company that provides Interferon Alfa 2b active pharmaceutical ingredient (API) or Interferon Alfa 2b finished formulations upon request. The Interferon Alfa 2b suppliers may include Interferon Alfa 2b API manufacturers, exporters, distributors and traders.
click here to find a list of Interferon Alfa 2b suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Interferon Alfa 2b DMF (Drug Master File) is a document detailing the whole manufacturing process of Interferon Alfa 2b active pharmaceutical ingredient (API) in detail. Different forms of Interferon Alfa 2b DMFs exist exist since differing nations have different regulations, such as Interferon Alfa 2b USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Interferon Alfa 2b DMF submitted to regulatory agencies in the US is known as a USDMF. Interferon Alfa 2b USDMF includes data on Interferon Alfa 2b's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Interferon Alfa 2b USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Interferon Alfa 2b suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Interferon Alfa 2b Drug Master File in Japan (Interferon Alfa 2b JDMF) empowers Interferon Alfa 2b API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Interferon Alfa 2b JDMF during the approval evaluation for pharmaceutical products. At the time of Interferon Alfa 2b JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Interferon Alfa 2b suppliers with JDMF on PharmaCompass.
Interferon Alfa 2b Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Interferon Alfa 2b GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Interferon Alfa 2b GMP manufacturer or Interferon Alfa 2b GMP API supplier for your needs.
A Interferon Alfa 2b CoA (Certificate of Analysis) is a formal document that attests to Interferon Alfa 2b's compliance with Interferon Alfa 2b specifications and serves as a tool for batch-level quality control.
Interferon Alfa 2b CoA mostly includes findings from lab analyses of a specific batch. For each Interferon Alfa 2b CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Interferon Alfa 2b may be tested according to a variety of international standards, such as European Pharmacopoeia (Interferon Alfa 2b EP), Interferon Alfa 2b JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Interferon Alfa 2b USP).
