
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
VMF
0
Australia

0
South Africa

0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
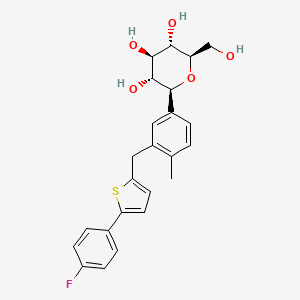

1. 1-(glucopyranosyl)-4-methyl-3-(5-(4-fluorophenyl)-2-thienylmethyl)benzene - T777973
2. Canagliflozin Hemihydrate
3. Canagliflozin, Anhydrous
4. Invokana
1. 842133-18-0
2. Canagliflozin Anhydrous
3. Ta-7284
4. Jnj-28431754
5. Jnj 24831754zae
6. Canagliflozin Hemihydrate
7. Canagliflozin [inn]
8. Canagliflozin Hydrate
9. (2s,3r,4r,5s,6r)-2-(3-((5-(4-fluorophenyl)thiophen-2-yl)methyl)-4-methylphenyl)-6-(hydroxymethyl)tetrahydro-2h-pyran-3,4,5-triol
10. Ta 7284
11. 1-(glucopyranosyl)-4-methyl-3-(5-(4-fluorophenyl)-2-thienylmethyl)benzene
12. Jnj 28431754
13. Chebi:73274
14. 6s49dgr869
15. (2s,3r,4r,5s,6r)-2-[3-[[5-(4-fluorophenyl)thiophen-2-yl]methyl]-4-methylphenyl]-6-(hydroxymethyl)oxane-3,4,5-triol
16. (1s)-1,5-anhydro-1-(3-((5-(4-fluorophenyl)-2-thienyl)methyl)-4-methylphenyl)-d-glucitol
17. (1s)-1,5-anhydro-1-(3-{[5-(4-fluorophenyl)-2-thienyl]methyl}-4-methylphenyl)-d-glucitol
18. (1s)-1,5-anhydro-1-c-[3-[[5-(4-fluorophenyl)-2-thienyl]methyl]-4-methylphenyl]-d-glucitol
19. D-glucitol,1,5-anhydro-1-c-[3-[[5-(4-fluorophenyl)-2-thienyl]methyl]-4-methylphenyl]-, (1s)-
20. (1s)-1,5-anhydro-1-c-(3-((5-(4-fluorophenyl)-2-thienyl)methyl)-4-methylphenyl)-d-glucitol
21. Unii-6s49dgr869
22. Jnj 24831754aaa
23. (2s,3r,4r,5s,6r)-2-(3-{[5-(4-fluorophenyl)thiophen-2-yl]methyl}-4-methylphenyl)-6-(hydroxymethyl)oxane-3,4,5-triol
24. Jnj 24831754
25. Canagliflozin [mi]
26. Mls006011126
27. Schembl157162
28. Canagliflozin [who-dd]
29. Gtpl4582
30. Chembl2048484
31. Hsdb 8284
32. Amy3291
33. Bcpp000303
34. Dtxsid601004469
35. Jnj 28431754aaa
36. Bdbm50386885
37. Mfcd18251436
38. S2760
39. Zinc43207238
40. Akos025401827
41. Bcp9000477
42. Ccg-229581
43. Cs-0522
44. Db08907
45. Ks-1443
46. Ncgc00346691-02
47. (1s)-1,5-anhydro-1-c-(3-((5-(4-fluorophenyl)thiophen-2-yl)methyl)-4-methylphenyl)-d-glucitol
48. Ac-26303
49. Hy-10451
50. Smr004702906
51. Sw219119-1
52. A25050
53. J-500391
54. Q5030940
55. (1s)-1,5-anhydro-1-(3-{[5-(4-fluorophenyl)-2-thienyl]methyl}-4-methyl-phenyl)-d-glucitol
56. (1s)-1,5-anhydro-1-c-(3-((5-(4-fluorophenyl)thiophen-2-yl)methyl)-4-methylphenyl)-d- Glucitol
57. D-glucitol, 1,5-anhydro-1-c-(3-((5-(4-fluorophenyl)-2-thienyl)methyl)-4- Methylphenyl)-, (1s)-
58. D-glucitol, 1,5-anhydro-1-c-(3-((5-(4-fluorophenyl)-2-thienyl)methyl)-4- Methylphenyl)-, (1s)-
59. D-glucitol, 1,5-anhydro-1-c-(3-((5-(4-fluorophenyl)-2-thienyl)methyl)-4-methylphenyl)-
60. Jnj24831754zae; Ta 7284;(2s,3r,4r,5s,6r)-2-(3-((5-(4-fluorophenyl)thiophen-2-yl)methyl)-4-methylphenyl)-6-(hydroxymethyl)-tetrahydro-2h-pyran-3,4,5-triol
| Molecular Weight | 444.5 g/mol |
|---|---|
| Molecular Formula | C24H25FO5S |
| XLogP3 | 3.2 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 5 |
| Exact Mass | 444.14067323 g/mol |
| Monoisotopic Mass | 444.14067323 g/mol |
| Topological Polar Surface Area | 118 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 574 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Invokana |
| PubMed Health | Canagliflozin (By mouth) |
| Drug Classes | Endocrine-Metabolic Agent, Hypoglycemic |
| Drug Label | INVOKANA (canagliflozin) contains canagliflozin, an inhibitor of sodium-glucose co-transporter 2 (SGLT2), the transporter responsible for reabsorbing the majority of glucose filtered by the kidney. Canagliflozin, the active ingredient of INVOKANA, is... |
| Active Ingredient | Canagliflozin |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 300mg; 100mg |
| Market Status | Prescription |
| Company | Janssen Pharms |
| 2 of 2 | |
|---|---|
| Drug Name | Invokana |
| PubMed Health | Canagliflozin (By mouth) |
| Drug Classes | Endocrine-Metabolic Agent, Hypoglycemic |
| Drug Label | INVOKANA (canagliflozin) contains canagliflozin, an inhibitor of sodium-glucose co-transporter 2 (SGLT2), the transporter responsible for reabsorbing the majority of glucose filtered by the kidney. Canagliflozin, the active ingredient of INVOKANA, is... |
| Active Ingredient | Canagliflozin |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 300mg; 100mg |
| Market Status | Prescription |
| Company | Janssen Pharms |
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Canagliflozin is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of September 30, 2015: https://clinicaltrials.gov/search/intervention=Canagliflozin
Invokana (canagliflozin) is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. /Included in US product label/
NIH; DailyMed. Current Medication Information for Invokana (Canagliflozin) Tablet, Film Coated (Updated: September 2015). Available from, as of November 20, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b9057d3b-b104-4f09-8a61-c61ef9d4a3f3
EXPL THER The proximal tubule's sodium-glucose linked transporter-2 (SGLT2) accounts for the vast majority of glucose reabsorption by the kidney. Its selective inhibition, accordingly, leads to substantial glycosuria, lowering blood glucose, and facilitating weight loss in individuals with diabetes. During the past year, two SGLT2 inhibitors, canagliflozin and dapagliflozin, have been approved for the treatment of type 2 diabetes. Beyond their anti-hyperglycemic properties, however, this new class of drugs has several other attributes that provide a theoretical basis for kidney protection. Like agents that block the renin-angiotensin system, SGLT2 inhibitors also reduce single-nephron glomerular filtration rate (SNGFR) in the chronically diseased kidney, though by quite different mechanisms. Additional potentially beneficial effects of SGLT2 inhibition include modest reductions in blood pressure and plasma uric acid. Finally, cell culture studies indicate that glucose uptake from the tubular lumen, as well as from the basolateral compartment, can contribute to proximal tubular production of extracellular matrix proteins. Whether such attributes will translate into reducing the progression of chronic kidney disease will require the undertaking of long-term, dedicated studies.
PMID:24257692 Gilbert RE; Kidney Int 86(4): 693-700 (2014).
EXPL THER Management of hypertension in diabetes is critical for reduction of cardiovascular mortality and morbidity. While blood pressure (BP) control has improved over the past two decades, the control rate is still well below 50% in the general population of patients with type 2 diabetes mellitus (T2DM). A new class of oral glucose-lowering agents has recently been approved; the sodium-glucose co-transporter 2 (SGLT2) inhibitors, which act by eliminating large amounts of glucose in the urine. Two agents, dapagliflozin and canagliflozin, are currently approved in the United States and Europe, and empagliflozin and ipragliflozin have reported Phase 3 trials. In addition to glucose lowering, SGLT2 inhibitors are associated with weight loss and act as osmotic diuretics, resulting in a lowering of BP. While not approved for BP-lowering, they may potentially aid BP goal achievement in people within 7-10 mm Hg of goal.
PMID:24631482 Oliva RV et al; J Am Soc Hypertens 8(5): 330-9.
Hypersensitivity reactions (e.g., generalized urticaria), some serious, have been reported with canagliflozin treatment. These reactions generally occurred within hours to days of canagliflozin initiation. If a hypersensitivity reaction occurs, the drug should be discontinued, appropriate treatment instituted, and the patient monitored until signs and symptoms resolve.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 3163
Dose-dependent increases in low-density lipoprotein (LDL)-cholesterol can occur during canagliflozin therapy. Serum LDL-cholesterol concentrations should be monitored during treatment with canagliflozin and such lipid elevations treated according to the standard of care.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 3162
When canagliflozin is added to therapy with an insulin secretagogue (e.g., a sulfonylurea) or insulin, the incidence of hypoglycemia is increased compared with sulfonylurea or insulin monotherapy. Therefore, patients receiving canagliflozin may require a reduced dosage of the concomitant insulin secretagogue or insulin to reduce the risk of hypoglycemia.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 3162
Canagliflozin may increase the risk of genital mycotic infections in males (e.g., balanoposthitis, candidal balanitis) and females (e.g., vulvovaginal candidiasis, vulvovaginal mycotic infection, vulvovaginitis). In clinical trials, patients with a history of genital mycotic infections and uncircumcised males were more likely to develop such infections. Patients should be monitored for genital mycotic infections and appropriate treatment should be instituted if these infections occur.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 3162
For more Drug Warnings (Complete) data for Canagliflozin (14 total), please visit the HSDB record page.
This drug is used in conjunction with diet and exercise to increase glycemic control in adults diagnosed with type 2 diabetes mellitus. Another indication for canagliflozin is the prevention of major cardiovascular events (myocardial infarction, stroke, or death due to a cardiovascular cause) in patients with type 2 diabetes, as well as hospitalization for heart failure in patients with type 2 diabetes[L5897,. In addition to the above, canagliflozin can be used to lower the risk of end-stage kidney disease and major increases in serum creatinine and cardiovascular death for patients with a combination of type 2 diabetes mellitus, diabetic nephropathy, and albuminuria. It is important to note that this drug is **not** indicated for the treatment of type 1 diabetes mellitus or diabetic ketoacidosis.
FDA Label
Invokana is indicated for the treatment of adults with insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exercise:
- as monotherapy when metformin is considered inappropriate due to intolerance or contraindications
- in addition to other medicinal products for the treatment of diabetes.
For study results with respect to combination of therapies, effects on glycaemic control, cardiovascular and renal events, and the populations studied, see sections 4. 4, 4. 5 and 5. 1.
Treatment of type II diabetes mellitus
This drug increases urinary glucose excretion and decreases the renal threshold for glucose (RTG) in a dose-dependent manner. The renal threshold is defined as the lowest level of blood glucose associated with the appearance of detectable glucose in the urine. The end result of canagliflozin administration is increased urinary excretion of glucose and less renal absorption of glucose, decreasing glucose concentration in the blood and improving glycemic control. **A note on type 2 diabetes and cardiovascular disease** The risk of cardiovascular events in diabetes type 2 is increased due to the damaging effects of diabetes on blood vessels and nerves in the cardiovascular system. In particular, there is a tendency for hyperglycemia to create pro-atherogenic (plaque forming) lesions in blood vessels, leading to various fatal and non-fatal events including stroke and myocardial infarction. Long-term glycemic control has been proven to be effective in the prevention of cardiovascular events such as myocardial infarction and stroke in patients with type 2 diabetes.
Sodium-Glucose Transporter 2 Inhibitors
Compounds that inhibit SODIUM-GLUCOSE TRANSPORTER 2. They lower blood sugar by preventing the reabsorption of glucose by the kidney and are used in the treatment of TYPE 2 DIABETES MELLITUS. (See all compounds classified as Sodium-Glucose Transporter 2 Inhibitors.)
A10BK02
A - Alimentary tract and metabolism
A10 - Drugs used in diabetes
A10B - Blood glucose lowering drugs, excl. insulins
A10BK - Sodium-glucose co-transporter 2 (sglt2) inhibitors
A10BK02 - Canagliflozin
Absorption
**Bioavailability and steady-state** The absolute oral bioavailability of canagliflozin, on average, is approximately 65%. Steady-state concentrations are achieved after 4 to 5 days of daily dose administration between the range of 100mg to 300mg. **Effect of food on absorption** Co-administration of a high-fat meal with canagliflozin exerted no appreciable effect on the pharmacokinetic parameters of canagliflozin. This drug may be administered without regard to food. Despite this, because of the potential of canagliflozin to decrease postprandial plasma glucose excretion due to prolonged intestinal glucose absorption, it is advisable to take this drug before the first meal of the day.
Route of Elimination
After a single oral radiolabeled dose canagliflozin dose to healthy subjects, the following ratios of canagliflozin or metabolites were measured in the feces and urine: **Feces** 41.5% as the unchanged radiolabeled drug 7.0% as a hydroxylated metabolite 3.2% as an O-glucuronide metabolite **Urine** About 33% of the ingested radiolabled dose was measured in the urine, generally in the form of O-glucuronide metabolites. Less than 1% of the dose was found excreted as unchanged drug in urine.
Volume of Distribution
This drug is extensively distributed throughout the body. On average, the volume of distribution of canagliflozin at steady state following a single intravenous dose in healthy patients was measured to be 83.5 L.
Clearance
In healthy subjects, canagliflozin clearance was approximately 192 mL/min after intravenous (IV) administration. The renal clearance of 100 mg and 300 mg doses of canagliflozin was measured to be in the range of 1.30 - 1.55 mL/min.
/MILK/ Canagliflozin is distributed into milk in rats; it is not known whether the drug is distributed into human milk.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 3163
Canagliflozin is an oral antihyperglycemic agent used for the treatment of type 2 diabetes mellitus. It blocks the reabsorption of glucose in the proximal renal tubule by inhibiting the sodium-glucose cotransporter 2. This article describes the in vivo biotransformation and disposition of canagliflozin after a single oral dose of [(14)C]canagliflozin to intact and bile duct-cannulated (BDC) mice and rats and to intact dogs and humans. Fecal excretion was the primary route of elimination of drug-derived radioactivity in both animals and humans. In BDC mice and rats, most radioactivity was excreted in bile. The extent of radioactivity excreted in urine as a percentage of the administered [(14)C]canagliflozin dose was 1.2%-7.6% in animals and approximately 33% in humans. The primary pathways contributing to the metabolic clearance of canagliflozin were oxidation in animals and direct glucuronidation of canagliflozin in humans. Unchanged canagliflozin was the major component in systemic circulation in all species. In human plasma, two pharmacologically inactive O-glucuronide conjugates of canagliflozin, M5 and M7, represented 19% and 14% of total drug-related exposure and were considered major human metabolites. Plasma concentrations of M5 and M7 in mice and rats from repeated dose safety studies were lower than those in humans given canagliflozin at the maximum recommended dose of 300 mg. However, biliary metabolite profiling in rodents indicated that mouse and rat livers had significant exposure to M5 and M7. Pharmacologic inactivity and high water solubility of M5 and M7 support glucuronidation of canagliflozin as a safe detoxification pathway.
PMID:24568888 Mamidi RN et al. Drug Metab Dispos 42(5): 903-16 (2014).
The mean absolute oral bioavailability of canagliflozin is approximately 65%. Co-administration of a high-fat meal with canagliflozin had no effect on the pharmacokinetics of canagliflozin; therefore, INVOKANA may be taken with or without food. However, based on the potential to reduce postprandial plasma glucose excursions due to delayed intestinal glucose absorption, it is recommended that INVOKANA be taken before the first meal of the day. The mean steady-state volume of distribution of canagliflozin following a single intravenous infusion in healthy subjects was 119 L, suggesting extensive tissue distribution. Canagliflozin is extensively bound to proteins in plasma (99%), mainly to albumin. Protein binding is independent of canagliflozin plasma concentrations. Plasma protein binding is not meaningfully altered in patients with renal or hepatic impairment. Following administration of a single oral [14C] canagliflozin dose to healthy subjects, 41.5%, 7.0%, and 3.2% of the administered radioactive dose was recovered in feces as canagliflozin, a hydroxylated metabolite, and an O-glucuronide metabolite, respectively. Enterohepatic circulation of canagliflozin was negligible. Approximately 33% of the administered radioactive dose was excreted in urine, mainly as O-glucuronide metabolites (30.5%). Less than 1% of the dose was excreted as unchanged canagliflozin in urine. Renal clearance of canagliflozin 100 mg and 300 mg doses ranged from 1.30 to 1.55 mL/min. Mean systemic clearance of canagliflozin was approximately 192 mL/min in healthy subjects following intravenous administration.
NIH; DailyMed. Current Medication Information for Invokana (Canagliflozin) Tablet, Film Coated (Updated: September 2015). Available from, as of November 20, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b9057d3b-b104-4f09-8a61-c61ef9d4a3f3
Canagliflozin is primarily metabolized by O-glucuronidation. It is mainly glucuronidated by UGT1A9 and UGT2B4 enzymes to two inactive O-glucuronide metabolites. The oxidative metabolism of canagliflozin by hepatic cytochrome enzyme CYP3A4 is negligible (about 7%) in humans.
O-glucuronidation is the major metabolic elimination pathway for canagliflozin, which is mainly glucuronidated by UGT1A9 and UGT2B4 to two inactive O-glucuronide metabolites. CYP3A4-mediated (oxidative) metabolism of canagliflozin is minimal (approximately 7%) in humans.
NIH; DailyMed. Current Medication Information for Invokana (Canagliflozin) Tablet, Film Coated (Updated: September 2015). Available from, as of November 20, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b9057d3b-b104-4f09-8a61-c61ef9d4a3f3
In a clinical study, the terminal half-life of canagliflozin was 10.6 hours for the 100mg dose and 13.1 hours for the 300 mg dose.
The sodium-glucose co-transporter2 (SGLT2), is found in the proximal tubules of the kidney, and reabsorbs filtered glucose from the renal tubular lumen. Canagliflozin inhibits the SGLT2 co-transporter. This inhibition leads to lower reabsorption of filtered glucose into the body and decreases the renal threshold for glucose (RTG), leading to increased glucose excretion in the urine.
Sodium-glucose co-transporter 2 (SGLT2), expressed in the proximal renal tubules, is responsible for the majority of the reabsorption of filtered glucose from the tubular lumen. Canagliflozin is an inhibitor of SGLT2. By inhibiting SGLT2, canagliflozin reduces reabsorption of filtered glucose and lowers the renal threshold for glucose (RTG), and thereby increases urinary glucose excretion (UGE).
NIH; DailyMed. Current Medication Information for Invokana (Canagliflozin) Tablet, Film Coated (Updated: September 2015). Available from, as of November 20, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b9057d3b-b104-4f09-8a61-c61ef9d4a3f3

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
47
PharmaCompass offers a list of Canagliflozin API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Canagliflozin manufacturer or Canagliflozin supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Canagliflozin manufacturer or Canagliflozin supplier.
PharmaCompass also assists you with knowing the Canagliflozin API Price utilized in the formulation of products. Canagliflozin API Price is not always fixed or binding as the Canagliflozin Price is obtained through a variety of data sources. The Canagliflozin Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Invokana manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Invokana, including repackagers and relabelers. The FDA regulates Invokana manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Invokana API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Invokana manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Invokana supplier is an individual or a company that provides Invokana active pharmaceutical ingredient (API) or Invokana finished formulations upon request. The Invokana suppliers may include Invokana API manufacturers, exporters, distributors and traders.
click here to find a list of Invokana suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Invokana DMF (Drug Master File) is a document detailing the whole manufacturing process of Invokana active pharmaceutical ingredient (API) in detail. Different forms of Invokana DMFs exist exist since differing nations have different regulations, such as Invokana USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Invokana DMF submitted to regulatory agencies in the US is known as a USDMF. Invokana USDMF includes data on Invokana's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Invokana USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Invokana suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Invokana Drug Master File in Korea (Invokana KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Invokana. The MFDS reviews the Invokana KDMF as part of the drug registration process and uses the information provided in the Invokana KDMF to evaluate the safety and efficacy of the drug.
After submitting a Invokana KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Invokana API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Invokana suppliers with KDMF on PharmaCompass.
A Invokana written confirmation (Invokana WC) is an official document issued by a regulatory agency to a Invokana manufacturer, verifying that the manufacturing facility of a Invokana active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Invokana APIs or Invokana finished pharmaceutical products to another nation, regulatory agencies frequently require a Invokana WC (written confirmation) as part of the regulatory process.
click here to find a list of Invokana suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Invokana as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Invokana API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Invokana as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Invokana and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Invokana NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Invokana suppliers with NDC on PharmaCompass.
Invokana Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Invokana GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Invokana GMP manufacturer or Invokana GMP API supplier for your needs.
A Invokana CoA (Certificate of Analysis) is a formal document that attests to Invokana's compliance with Invokana specifications and serves as a tool for batch-level quality control.
Invokana CoA mostly includes findings from lab analyses of a specific batch. For each Invokana CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Invokana may be tested according to a variety of international standards, such as European Pharmacopoeia (Invokana EP), Invokana JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Invokana USP).
