
Synopsis
Synopsis
0
EU WC
0
VMF
0
Australia

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
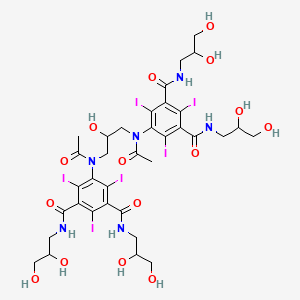

1. Contrast Media 2-5410-3
2. Iodixanol-320
3. Visipaque
4. Visipaque Unique Softpac
1. 92339-11-2
2. Visipaque
3. Indixanol
4. Iodixanolum
5. 2-5410-3a
6. Hw8w27htxx
7. Nsc-760069
8. 5,5'-((2-hydroxytrimethylene)bis(acetylimino))bis(n,n'-bis(2,3-dihydroxypropyl)-2,4,6-triiodoisophthalamide)
9. Chebi:31705
10. 5-[acetyl-[3-[n-acetyl-3,5-bis(2,3-dihydroxypropylcarbamoyl)-2,4,6-triiodoanilino]-2-hydroxypropyl]amino]-1-n,3-n-bis(2,3-dihydroxypropyl)-2,4,6-triiodobenzene-1,3-dicarboxamide
11. 2-541o-3a
12. Dsstox_cid_25523
13. Dsstox_rid_80929
14. Dsstox_gsid_45523
15. 1,3-benzenedicarboxamide, 5,5'-((2-hydroxy-1,3-propanediyl)bis(acetylimino))bis(n,n'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-
16. 5-[acetyl-[3-[n-acetyl-3,5-bis[[(2s)-2,3-dihydroxypropyl]carbamoyl]-2,4,6-triiodoanilino]-2-hydroxypropyl]amino]-1-n,3-n-bis[(2s)-2,3-dihydroxypropyl]-2,4,6-triiodobenzene-1,3-dicarboxamide
17. Optiprep; Visipaque; Visipaque 270;2-5410-3a
18. Visipaque 270
19. Visipaque 320
20. Iodixanolum [latin]
21. Optiprep
22. 1,3-benzenedicarboxamide, 5,5'-[(2-hydroxy-1,3-propanediyl)bis(acetylimino)]bis[n,n'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-
23. 5,5'-((2-hydroxypropane-1,3-diyl)bis(acetylazanediyl))bis(n1,n3-bis(2,3-dihydroxypropyl)-2,4,6-triiodoisophthalamide)
24. Ccris 7567
25. Sr-01000872678
26. Unii-hw8w27htxx
27. Hsdb 8076
28. Iodixanol [usan:usp:inn:ban]
29. Visipaque (tn)
30. Ncgc00016958-01
31. Cas-92339-11-2
32. Visipaque;iodixanolum
33. Iodixanol [inn]
34. Iodixanol [jan]
35. Iodixanol [mi]
36. Iodixanol [usan]
37. Prestwick0_000848
38. Prestwick1_000848
39. Prestwick2_000848
40. Prestwick3_000848
41. Iodixanol [vandf]
42. Iodixanol [mart.]
43. Ec 618-837-0
44. Iodixanol [usp-rs]
45. Iodixanol [who-dd]
46. Iodixanol (jan/usp/inn)
47. Schembl21546
48. Bspbio_000835
49. Spbio_002756
50. Bpbio1_000919
51. Iodixanol [orange Book]
52. Chembl1200507
53. Dtxsid2045523
54. Iodixanol [ep Monograph]
55. Iodixanol [usp Impurity]
56. Iodixanol [usp Monograph]
57. Hms1570j17
58. Hms2097j17
59. Hms3714j17
60. Pharmakon1600-01503835
61. Bcp11111
62. Hy-b1426
63. Tox21_110711
64. Nsc760069
65. S5218
66. Akos015895607
67. Tox21_110711_1
68. Ac-7610
69. Ccg-213207
70. Db01249
71. Nsc 760069
72. Ncgc00179408-01
73. Ncgc00179408-03
74. 5-(acetyl-(3-(acetyl-(3,5-bis(2,3-dihydroxypropylcarbamoyl)-2,4,6-triiodophenyl)amino)-2- Hydroxypropyl)amino)-n,n'-bis(2,3-dihydroxypropyl)-2,4,6-triiodobenzene-1,3-dicarboxamide; 5,5'-((2-hydroxytrimethylene)bis(acetylimino))bis(n,n'-bis(2,3-dihydroxypropyl)-2,4,6- Triiodoisophthalamide);
75. Ab00513936
76. Cs-0013137
77. Ft-0627254
78. Ft-0645028
79. D01474
80. Ab00513936_02
81. 339i112
82. A844203
83. Q2419291
84. Sr-01000872678-1
85. Sr-01000872678-2
86. Brd-a08660406-001-01-8
87. Optiprep(tm) Density Gradient Medium, Used For Cell And Subcellular Organelle Isolation
88. 5,5'-(2-hydroxypropane-1,3-diyl)bis(acetylazanediyl)bis(n1,n3-bis(2,3-dihydroxypropyl)-2,4,6-triiodoisophthalamide)
89. 5,5'-[(2-hydroxypropane-1,3-diyl)bis(acetylimino)]bis[n,n'-bis(2,3-dihydroxypropyl)-2,4,6-triiodobenzene-1,3-dicarboxamide]
90. 5,5-[(2-hydroxy-1,3-propanediyl)bis(acetylimino)]bis[n,n-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzenedicarboxamide
91. 5-[[3-[[3,5-bis[2,3-bis(oxidanyl)propylcarbamoyl]-2,4,6-tris(iodanyl)phenyl]-ethanoyl-amino]-2-oxidanyl-propyl]-ethanoyl-amino]-n1,n3-bis[2,3-bis(oxidanyl)propyl]-2,4,6-tris(iodanyl)benzene-1,3-dicarboxamide
92. 5-[acetyl-[3-[n-acetyl-3,5-bis[(2,3-dihydroxypropylamino)-oxomethyl]-2,4,6-triiodoanilino]-2-hydroxypropyl]amino]-n1,n3-bis(2,3-dihydroxypropyl)-2,4,6-triiodobenzene-1,3-dicarboxamide
93. 5-{n-[3-(n-{3,5-bis[(2,3-dihydroxypropyl)carbamoyl]-2,4,6-triiodophenyl}acetamido)-2-hydroxypropyl]acetamido}-1-n,3-n-bis(2,3-dihydroxypropyl)-2,4,6-triiodobenzene-1,3-dicarboxamide
| Molecular Weight | 1550.2 g/mol |
|---|---|
| Molecular Formula | C35H44I6N6O15 |
| XLogP3 | -3.4 |
| Hydrogen Bond Donor Count | 13 |
| Hydrogen Bond Acceptor Count | 15 |
| Rotatable Bond Count | 22 |
| Exact Mass | 1549.7133 g/mol |
| Monoisotopic Mass | 1549.7133 g/mol |
| Topological Polar Surface Area | 339 Ų |
| Heavy Atom Count | 62 |
| Formal Charge | 0 |
| Complexity | 1330 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 4 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Visipaque 270 |
| Active Ingredient | Iodixanol |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 55% |
| Market Status | Prescription |
| Company | Ge Healthcare |
| 2 of 4 | |
|---|---|
| Drug Name | Visipaque 320 |
| Active Ingredient | Iodixanol |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 65.2% |
| Market Status | Prescription |
| Company | Ge Healthcare |
| 3 of 4 | |
|---|---|
| Drug Name | Visipaque 270 |
| Active Ingredient | Iodixanol |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 55% |
| Market Status | Prescription |
| Company | Ge Healthcare |
| 4 of 4 | |
|---|---|
| Drug Name | Visipaque 320 |
| Active Ingredient | Iodixanol |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 65.2% |
| Market Status | Prescription |
| Company | Ge Healthcare |
Iodixanol is indicated for contrast enhanced computerized tomography imaging of the head and body, excretory urography, and peripheral venography. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for VISIPAQUE (iodixanol) injection, solution (May 2009). Available from, as of July 11, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=2d964c13-88ad-43be-88d4-a3a7ea4d5b5e
Iodixanol is indicated for angiocardiography (left ventriculography and selective coronary arteriography), peripheral arteriography, visceral arteriography, and cerebral arteriography. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for VISIPAQUE (iodixanol) injection, solution (May 2009). Available from, as of July 11, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=2d964c13-88ad-43be-88d4-a3a7ea4d5b5e
Iodixanol is indicated for intra-arterial digital subtraction angiography. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for VISIPAQUE (iodixanol) injection, solution (May 2009). Available from, as of July 11, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=2d964c13-88ad-43be-88d4-a3a7ea4d5b5e
Iodixanol is indicated for contrast enhanced computerized tomography imaging of the head and body, and excretory urography. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for VISIPAQUE (iodixanol) injection, solution (May 2009). Available from, as of July 11, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=2d964c13-88ad-43be-88d4-a3a7ea4d5b5e
For more Therapeutic Uses (Complete) data for Iodixanol (6 total), please visit the HSDB record page.
The following selected adverse events were reported in ?0.5% of the 1244 patients in controlled clinical trials who received iodixanol injection: General Disorders: back pain, fatigue, malaise; Cardiovascular Disorders: arrhythmias, cardiac failure, conduction abnormalities, hypotension, myocardial infarction; Nervous System: cerebral vascular disorder, convulsions, hypoesthesia, stupor, confusion; Gastrointestinal System Disorders: dyspepsia; Hypersensitivity Disorders: pharyngeal edema; Respiratory System Disorders: asthma, bronchitis, dyspnea, pulmonary edema, rhinitis; Renal System Disorders: abnormal renal function, acute renal failure, hematuria; Peripheral Vascular Disorders: flushing, peripheral ischemia; Skin and Appendage Disorders: hematoma, increased sweating; Special Senses, Other Disorders: tinnitus; Vision Disorders: abnormal vision.
US Natl Inst Health; DailyMed. Current Medication Information for VISIPAQUE (iodixanol) injection, solution (May 2009). Available from, as of July 11, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=2d964c13-88ad-43be-88d4-a3a7ea4d5b5e
Additional adverse events reported in other clinical studies and in foreign postmarketing surveillance and foreign clinical trials with the use of iodixanol injection are: anaphylactic reactions, anaphylactoid reactions, hypoglycemia, amnesia, cardiac arrest, hypertension, dyskinesia, hemorrhage not otherwise specified, polymyalgia rheumatica, pulmonary embolism, respiratory depression, and cortical blindness.
US Natl Inst Health; DailyMed. Current Medication Information for VISIPAQUE (iodixanol) injection, solution (May 2009). Available from, as of July 11, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=2d964c13-88ad-43be-88d4-a3a7ea4d5b5e
... Selected commonly reported adverse events in pediatrics include: vomiting, nausea, fever, rash, and pruritus. Less frequently reported events are apnea, disseminated intravascular coagulation, atrioventricular block and bundle branch block, arrhythmia, cardiac failure, renal failure and taste perversion.
US Natl Inst Health; DailyMed. Current Medication Information for VISIPAQUE (iodixanol) injection, solution (May 2009). Available from, as of July 11, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=2d964c13-88ad-43be-88d4-a3a7ea4d5b5e
In patients with normal blood-brain barriers and renal failure, iodinated contrast agents have been associated with blood-brain barrier disruption and accumulation of contrast in the brain.
US Natl Inst Health; DailyMed. Current Medication Information for VISIPAQUE (iodixanol) injection, solution (May 2009). Available from, as of July 11, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=2d964c13-88ad-43be-88d4-a3a7ea4d5b5e
For more Drug Warnings (Complete) data for Iodixanol (28 total), please visit the HSDB record page.
Iodixanol is a contrast agent during coronary angiography.
FDA Label
Iodixanol is a contrast agent commonly used during coronary angiography, particularly in individuals with renal dysfunction, as it is believed to be less toxic to the kidneys than most other intravascular contrast agents. It is an imaging contrast agent with the same osmolality as blood (290mOsm/kg H20).
Contrast Media
Substances used to allow enhanced visualization of tissues. (See all compounds classified as Contrast Media.)
V08AB09
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
V - Various
V08 - Contrast media
V08A - X-ray contrast media, iodinated
V08AB - Watersoluble, nephrotropic, low osmolar x-ray contrast media
V08AB09 - Iodixanol
Route of Elimination
In adults, approximately 97% of the injected dose of iodixanol is excreted unchanged in urine within 24 hours, with less than 2% excreted in feces within five days post-injection.
Volume of Distribution
0.26 L/kg
Absorption and excretion of iodixanol 320 mg I/ml were investigated in rats after intragastric administration of 2.5 g I/kg b.w. Animals were observed for up to 96 hours after treatment, and blood, urine and feces taken at several time-points throughout the experiment. Concentrations of iodixanol in serum and urine were measured by means of reversed-phase high-performance liquid chromatography. Fecal concentrations of iodixanol, based on iodine measurements, were determined by X-ray fluorescence spectrometry. Serial radiographs were obtained and histopathological examination was performed on selected tissues. The results indicate that less than 1% of the intragastric dose of iodixanol is absorbed from the intestine into the blood stream. No adverse clinical signs were observed, and there were no treatment-related histomorphological findings.
PMID:8610524 Dunkel JA et al; Acta Radiol Suppl 399: 253-7 (1995)
In 40 healthy, young male volunteers receiving a single intravenous administration of iodixanol injection in doses of 0.3 to 1.2 gI/kg body weight, ... renal clearance was 110 mL/min (+/-14), equivalent to glomerular filtration (108 mL/min). These values were independent of the dose administered.
US Natl Inst Health; DailyMed. Current Medication Information for VISIPAQUE (iodixanol) injection, solution (May 2009). Available from, as of July 11, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=2d964c13-88ad-43be-88d4-a3a7ea4d5b5e
... In a study of 16 adult patients who were scheduled for renal transplant, the elimination of iodixanol 320 mgI/mL was studied. The patients' baseline mean creatinine levels were 6.3 mg/dL (+/-1.5) and mean creatinine clearances were 13.61 mL/min (+/- 4.67). ... In these patients, levels of iodixanol were detected 5 days after dosing. Contrast enhancement time in kidneys increased from 6 hours to at least 24 hours. ...
US Natl Inst Health; DailyMed. Current Medication Information for VISIPAQUE (iodixanol) injection, solution (May 2009). Available from, as of July 11, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=2d964c13-88ad-43be-88d4-a3a7ea4d5b5e
Plasma and urine levels suggest that body clearance of iodixanol is primarily due to renal clearance. In adults, approximately 97% of the injected dose of iodixanol is excreted unchanged in urine within 24 hours, with less than 2% excreted in feces within five days post-injection.
US Natl Inst Health; DailyMed. Current Medication Information for VISIPAQUE (iodixanol) injection, solution (May 2009). Available from, as of July 11, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=2d964c13-88ad-43be-88d4-a3a7ea4d5b5e
For more Absorption, Distribution and Excretion (Complete) data for Iodixanol (14 total), please visit the HSDB record page.
Excreted unchanged
Iodixanol metabolites have not been demonstrated.
US Natl Inst Health; DailyMed. Current Medication Information for VISIPAQUE (iodixanol) injection, solution (May 2009). Available from, as of July 11, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=2d964c13-88ad-43be-88d4-a3a7ea4d5b5e
2.1 hours. In patients with significantly impaired renal function (mean creatinine clearance rate, 9.91 [± 3.58] mL per minute), the plasma half-life is increased to 23 hours.
Forty pediatric patients < or = 12 years old, with renal function that is normal for their age, received multiple intra-arterial administrations of iodixanol injection in doses of 0.32 to 3.2 gI/kg body weight. The elimination half-lives for these patients are ...: 0.185 hr -1 (newborn to 2 months old), 0.256 hr -1 (2 to <6 months old), 0.299 hr -1 (6 months to<1 year), 0.322 hr -1 (1 to<2 years), and 0.307 hr -1 (2 to < or = 12 years old). The adult mean terminal elimination rate constant is 0.336 hr-1. ...
US Natl Inst Health; DailyMed. Current Medication Information for VISIPAQUE (iodixanol) injection, solution (May 2009). Available from, as of July 11, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=2d964c13-88ad-43be-88d4-a3a7ea4d5b5e
In 40 healthy, young male volunteers receiving a single intravenous administration of iodixanol injection in doses of 0.3 to 1.2 gI/kg body weight, the elimination half-life was 2.1 hr (+/- 0.1) ...
US Natl Inst Health; DailyMed. Current Medication Information for VISIPAQUE (iodixanol) injection, solution (May 2009). Available from, as of July 11, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=2d964c13-88ad-43be-88d4-a3a7ea4d5b5e
... Iodixanol was rapidly excreted, mainly via the kidneys, with a plasma half-life in rats and monkeys of 25 and 76 mins, respectively. ...
PMID:8610532 Heglund IF et al; Acta Radiol Suppl 399: 69-82 (1995)
... In a study of 16 adult patients who were scheduled for renal transplant, the elimination of iodixanol 320 mgI/mL was studied. ... In these patients, the plasma half-life was increased to 23 hours (normal t1/2 = 2 hours). ...
US Natl Inst Health; DailyMed. Current Medication Information for VISIPAQUE (iodixanol) injection, solution (May 2009). Available from, as of July 11, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=2d964c13-88ad-43be-88d4-a3a7ea4d5b5e
Organic iodine compounds attenuate x-rays as they pass through the body, thereby allowing the body structures containing iodine to be delineated in contrast to those structures that do not contain iodine. The degree of opacity produced by these compounds is directly proportional to the total amount (concentration and volume) of the iodinated contrast agent in the path of the x-rays. After intravascular administration, iodixanol makes opaque those internal structures in its path of flow, allowing their visualization until significant hemodilution and elimination occur.
Intravascular injection of iodixanol opacifies those vessels in the path of flow of the contrast agent, permitting radiographic visualization of the internal structures until significant dilution and elimination occurs.
US Natl Inst Health; DailyMed. Current Medication Information for VISIPAQUE (iodixanol) injection, solution (May 2009). Available from, as of July 11, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=2d964c13-88ad-43be-88d4-a3a7ea4d5b5e
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
46
PharmaCompass offers a list of Iodixanol API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Iodixanol manufacturer or Iodixanol supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Iodixanol manufacturer or Iodixanol supplier.
PharmaCompass also assists you with knowing the Iodixanol API Price utilized in the formulation of products. Iodixanol API Price is not always fixed or binding as the Iodixanol Price is obtained through a variety of data sources. The Iodixanol Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Iodixanol manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Iodixanol, including repackagers and relabelers. The FDA regulates Iodixanol manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Iodixanol API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Iodixanol manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Iodixanol supplier is an individual or a company that provides Iodixanol active pharmaceutical ingredient (API) or Iodixanol finished formulations upon request. The Iodixanol suppliers may include Iodixanol API manufacturers, exporters, distributors and traders.
click here to find a list of Iodixanol suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Iodixanol DMF (Drug Master File) is a document detailing the whole manufacturing process of Iodixanol active pharmaceutical ingredient (API) in detail. Different forms of Iodixanol DMFs exist exist since differing nations have different regulations, such as Iodixanol USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Iodixanol DMF submitted to regulatory agencies in the US is known as a USDMF. Iodixanol USDMF includes data on Iodixanol's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Iodixanol USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Iodixanol suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Iodixanol Drug Master File in Japan (Iodixanol JDMF) empowers Iodixanol API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Iodixanol JDMF during the approval evaluation for pharmaceutical products. At the time of Iodixanol JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Iodixanol suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Iodixanol Drug Master File in Korea (Iodixanol KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Iodixanol. The MFDS reviews the Iodixanol KDMF as part of the drug registration process and uses the information provided in the Iodixanol KDMF to evaluate the safety and efficacy of the drug.
After submitting a Iodixanol KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Iodixanol API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Iodixanol suppliers with KDMF on PharmaCompass.
A Iodixanol CEP of the European Pharmacopoeia monograph is often referred to as a Iodixanol Certificate of Suitability (COS). The purpose of a Iodixanol CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Iodixanol EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Iodixanol to their clients by showing that a Iodixanol CEP has been issued for it. The manufacturer submits a Iodixanol CEP (COS) as part of the market authorization procedure, and it takes on the role of a Iodixanol CEP holder for the record. Additionally, the data presented in the Iodixanol CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Iodixanol DMF.
A Iodixanol CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Iodixanol CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Iodixanol suppliers with CEP (COS) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Iodixanol as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Iodixanol API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Iodixanol as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Iodixanol and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Iodixanol NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Iodixanol suppliers with NDC on PharmaCompass.
Iodixanol Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Iodixanol GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Iodixanol GMP manufacturer or Iodixanol GMP API supplier for your needs.
A Iodixanol CoA (Certificate of Analysis) is a formal document that attests to Iodixanol's compliance with Iodixanol specifications and serves as a tool for batch-level quality control.
Iodixanol CoA mostly includes findings from lab analyses of a specific batch. For each Iodixanol CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Iodixanol may be tested according to a variety of international standards, such as European Pharmacopoeia (Iodixanol EP), Iodixanol JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Iodixanol USP).
