
Synopsis
Synopsis
0
EU WC
0
VMF
0
Australia

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
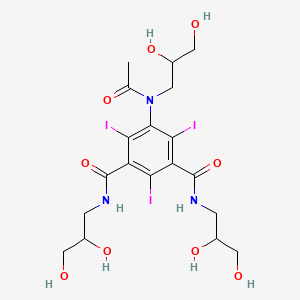

1. Compound 545
2. Exypaque
3. Iohexol 350
4. Nycodenz
5. Omnipaque
1. 66108-95-0
2. Omnipaque
3. Nycodenz
4. Exypaque
5. Iohexolum
6. Omnipaque 240
7. Omnipaque 70
8. Omnipaque 140
9. Omnipaque 180
10. Omnipaque 210
11. Omnipaque 300
12. Omnipaque 350
13. Win 39424
14. Oraltag
15. Win-39424
16. Chebi:31709
17. N,n'-bis(2,3-dihydroxypropyl)-5-(n-(2,3-dihydroxypropyl)acetamido)-2,4,6-triiodoisophthalamide
18. 5-[acetyl(2,3-dihydroxypropyl)amino]-1-n,3-n-bis(2,3-dihydroxypropyl)-2,4,6-triiodobenzene-1,3-dicarboxamide
19. 5-[acetyl(2,3-dihydroxypropyl)amino]-n,n'-bis(2,3-dihydroxypropyl)-2,4,6-triiodoisophthalamide
20. N1,n3-bis(2,3-dihydroxypropyl)-5-(n-(2,3-dihydroxypropyl)acetamido)-2,4,6-triiodoisophthalamide
21. 4419t9mx03
22. Nsc-759636
23. Ncgc00166000-01
24. Dsstox_cid_3157
25. 5-(n-2,3-dihydroxypropylacetamido)-2,4,6-triiodo-n,n'-bis(2,3-dihydroxypropyl)isophthalamide
26. Dsstox_rid_76895
27. Dsstox_gsid_23157
28. 1,3-benzenedicarboxamide, 5-(acetyl(2,3-dihydroxypropyl)amino)-n,n'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-
29. Histodenz
30. Iohexolum [inn-latin]
31. 1,3-benzenedicarboxamide, 5-[acetyl(2,3-dihydroxypropyl)amino]-n1,n3-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-
32. 5-[acetyl(2,3-dihydroxypropyl)amino]-n,n'-bis(2,3-dihydroxypropyl)-2,4,6-triiodobenzene-1,3-dicarboxamide
33. 5-[n-(2,3-dihydroxypropyl)acetamido]-2,4,6-triiodo-n,n'-bis(2,3-dihydroxypropyl)isophthalamide
34. Smr000857075
35. Einecs 266-164-2
36. Brn 2406632
37. Unii-4419t9mx03
38. Omnipaque (tn)
39. 1,3-benzenedicarboxamide, 5-[acetyl(2,3-dihydroxypropyl)amino]-n,n'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-
40. N,n'-bis(2,3-dihydroxypropyl)-5-[n-(2,3-dihydroxypropyl)acetamido]-2,4,6-triiodoisophthalamide
41. Prestwick_802
42. Cas-66108-95-0
43. Iohexol [usan:usp:inn:ban:jan]
44. Iohexol [vandf]
45. Iohexol [usan]
46. Iohexol [inn]
47. Iohexol [jan]
48. Iohexol [mi]
49. Iohexol [mart.]
50. Prestwick0_000512
51. Prestwick1_000512
52. Prestwick2_000512
53. Prestwick3_000512
54. Iohexol [usp-rs]
55. Iohexol [who-dd]
56. Iohexol [who-ip]
57. Nycodenz;omnipaque;exypaque
58. Ec 266-164-2
59. Iohexol, Analytical Standard
60. Schembl26501
61. Bspbio_000463
62. Mls001332585
63. Mls001332586
64. Mls002153854
65. Iohexol (jp17/usp/inn)
66. Iohexol [ep Impurity]
67. Iohexol [orange Book]
68. Spbio_002384
69. Iohexol [ep Monograph]
70. Bpbio1_000511
71. Iohexol [usp Monograph]
72. Chembl1200455
73. Dtxsid6023157
74. Bcbcmap01_000051
75. Iohexolum [who-ip Latin]
76. Hms1569h05
77. Hms2096h05
78. Hms2235d07
79. Hms3369o04
80. Hms3713h05
81. Albb-028959
82. Amy21804
83. Bcp31800
84. Hy-b0594
85. Tox21_112286
86. Bdbm50247977
87. Iohexol (mixture Of Isomers)
88. Mfcd00077732
89. S4531
90. Akos015895399
91. Tox21_112286_1
92. Ac-1934
93. Ccg-220512
94. Db01362
95. Nsc 759636
96. Smp1_000152
97. Ncgc00166000-02
98. Ncgc00166000-04
99. As-12699
100. Ft-0627276
101. I0903
102. D01817
103. D91214
104. Histodenz(tm), Nonionic Density Gradient Medium
105. 108i950
106. A835339
107. Q410683
108. Sr-01000838892
109. Sr-01000838892-2
110. Iohexol, European Pharmacopoeia (ep) Reference Standard
111. Iohexol, United States Pharmacopeia (usp) Reference Standard
112. 5-(n-dhp-acetamido)-2,4,6-triiodo-n,n'-b Is-dhp-isophthalami
113. Iohexol, Pharmaceutical Secondary Standard; Certified Reference Material
114. Iohexol For Peak Identification, European Pharmacopoeia (ep) Reference Standard
115. 1,3-benzenedicarboxamide, 5-(acetyl(2,3-dihydroxypropyl)amino)-n,n'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo
116. 1-n,3-n-bis(2,3-dihydroxypropyl)-5-[n-(2,3-dihydroxypropyl)acetamido]-2,4,6-triiodobenzene-1,3-dicarboxamide
117. 5-[acetyl(2,3-dihydroxypropyl)amino]-n1,n3-bis(2,3-dihydroxypropyl)-2,4,6-triiodobenzene-1,3-dicarboxamide
118. N1,n3-bis(2,3-dihydroxypropyl)-5-[n-(2,3-dihydroxypropyl)acetamido]-2,4,6-triiodobenzene-1,3-dicarboxamide
119. N1,n3-bis[2,3-bis(oxidanyl)propyl]-5-[2,3-bis(oxidanyl)propyl-ethanoyl-amino]-2,4,6-tris(iodanyl)benzene-1,3-dicarboxamide
| Molecular Weight | 821.1 g/mol |
|---|---|
| Molecular Formula | C19H26I3N3O9 |
| XLogP3 | -3 |
| Hydrogen Bond Donor Count | 8 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 12 |
| Exact Mass | 820.8803 g/mol |
| Monoisotopic Mass | 820.8803 g/mol |
| Topological Polar Surface Area | 200 Ų |
| Heavy Atom Count | 34 |
| Formal Charge | 0 |
| Complexity | 653 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 3 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Omnipaque 180 |
| PubMed Health | Iohexol (Injection) |
| Drug Classes | Radiological Non-Ionic Contrast Media |
| Active Ingredient | Iohexol |
| Dosage Form | Solution |
| Route | Injection, oral, rectal |
| Strength | 38.8% |
| Market Status | Prescription |
| Company | Ge Healthcare |
| 2 of 4 | |
|---|---|
| Drug Name | Omnipaque 240 |
| Active Ingredient | Iohexol |
| Dosage Form | Solution |
| Route | Injection, oral, rectal |
| Strength | 51.8% |
| Market Status | Prescription |
| Company | Ge Healthcare |
| 3 of 4 | |
|---|---|
| Drug Name | Omnipaque 180 |
| PubMed Health | Iohexol (Injection) |
| Drug Classes | Radiological Non-Ionic Contrast Media |
| Active Ingredient | Iohexol |
| Dosage Form | Solution |
| Route | Injection, oral, rectal |
| Strength | 38.8% |
| Market Status | Prescription |
| Company | Ge Healthcare |
| 4 of 4 | |
|---|---|
| Drug Name | Omnipaque 240 |
| Active Ingredient | Iohexol |
| Dosage Form | Solution |
| Route | Injection, oral, rectal |
| Strength | 51.8% |
| Market Status | Prescription |
| Company | Ge Healthcare |
Iohexol ia used in myelography, arthrography, nephroangiography, arteriography, and other radiographic procedures.
Iohexol is an effective non-ionic, water-soluble contrast agent which is used in myelography, arthrography, nephroangiography, arteriography, and other radiographic procedures. Its low systemic toxicity is the combined result of low chemotoxicity and low osmolality.
Contrast Media
Substances used to allow enhanced visualization of tissues. (See all compounds classified as Contrast Media.)
V08AB02
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
V - Various
V08 - Contrast media
V08A - X-ray contrast media, iodinated
V08AB - Watersoluble, nephrotropic, low osmolar x-ray contrast media
V08AB02 - Iohexol
Absorption
Small amounts are absorbed through the bladder via intravesical instillation. Following intrauterine instillation, the majority of the medium within the uterine cavity is discharged into the vagina immediately upon termination of procedure. However, any medium retained in the uterine or peritoneal cavity is absorbed systemically within 60 minutes. May not be absorbed for up to 24 hours if tubes are obstructed and dilated.
Route of Elimination
Iohexol is absorbed from cerebrospinal fluid (CSF) into the bloodstream and is eliminated by renal excretion. No significant metabolism, deiodination, or biotransformation occurs.
Volume of Distribution
350-849 mL/kg
Clearance
109 mL/min [Adult patients receiving 16-18 ml of iohexol (180 mgI/mL) by lumbar intrathecal injection]
Intrathecal half-life is 3.4 hours (mean). Intravascular is approximately 2 hours (with normal renal function).
Organic iodine compounds block x-rays as they pass through the body, thereby allowing body structures containing iodine to be delineated in contrast to those structures that do not contain iodine. The degree of opacity produced by these compounds is directly proportional to the total amount (concentration and volume) of the iodinated contrast agent in the path of the x-rays. After intrathecal administration into the subarachnoid space, diffusion of iohexol in the CSF allows the visualization of the subarachnoid spaces of the head and spinal canal. After intravascular administration, iohexol makes opaque those vessels in its path of flow, allowing visualization of the internal structures until significant hemodilution occurs.
GDUFA
DMF Review : Complete
Rev. Date : 2023-05-03
Pay. Date : 2023-03-23
DMF Number : 38195
Submission : 2023-03-20
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 23148
Submission : 2009-10-02
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 26641
Submission : 2013-01-24
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 22535
Submission : 2009-02-23
Status : Inactive
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2021-10-13
Pay. Date : 2021-09-14
DMF Number : 36038
Submission : 2021-09-15
Status : Active
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Registration Number : 305MF10075
Registrant's Address : No. 01 Pengze Nine road, Jishan Industrial Park, Pengze County, Jiujiang City, Jiangx...
Initial Date of Registration : 2023-07-05
Latest Date of Registration : 2023-07-05

Registration Number : 217MF10959
Registrant's Address : Lindesnesveien 208, 4521 Lindesnes, Norway
Initial Date of Registration : 2005-11-14
Latest Date of Registration : 2023-02-02

Registration Number : 304MF10054
Registrant's Address : No. 1 Starry Road of Xianju Modern Industrial Centralization Zone, Xianju, Zhejiang, ...
Initial Date of Registration : 2022-03-16
Latest Date of Registration : 2022-03-16

Registration Number : 219MF10263
Registrant's Address : Zhejiang Province Chemical and Medical Materials Base, Linhai Park
Initial Date of Registration : 2007-08-16
Latest Date of Registration : 2014-07-01

Registration Number : 227MF10106
Registrant's Address : Zhejiang Province Chemical and Medical Materials Base, Linhai Park
Initial Date of Registration : 2015-04-09
Latest Date of Registration : 2015-04-09

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Registrant Name : Parmarine Co., Ltd.
Registration Date : 2022-05-17
Registration Number : 20220517-210-J-1297
Manufacturer Name : Divi's Laboratories Ltd.
Manufacturer Address : Unit-2, Chippada Village, Annavaram Post – 531 162, Bheemunipatnam Mandal, Visakhap...

Registrant Name : Korea Biochem Pharmaceutical Co., Ltd.
Registration Date : 2020-07-17
Registration Number : 20200107-210-J-363(2)
Manufacturer Name : GE Healthcare AS
Manufacturer Address : Lindesnesveien 208, Lindesnes, NO-4521, Norway

Registrant Name : Palm East Co., Ltd.
Registration Date : 2020-04-02
Registration Number : 20200107-210-J-363(1)
Manufacturer Name : GE Healthcare AS
Manufacturer Address : Lindesnesveien 208, Lindesnes, NO-4521, Norway

Registrant Name : GE Healthcare AS Korea Branch
Registration Date : 2020-01-07
Registration Number : 20200107-210-J-363
Manufacturer Name : GE Healthcare AS, Lindesnes ...
Manufacturer Address : Lindesnesveien 208, NO 4521, Lindesnes, Norway

Registrant Name : Pharmapia Co., Ltd.
Registration Date : 2021-02-15
Registration Number : 20210215-210-J-472
Manufacturer Name : Zhejiang Haizhou Pharmaceuti...
Manufacturer Address : Yanhai Industrial Zone, Linhai City, Zhejiang Province, China

Registrant Name : Ace Biopharm Co., Ltd.
Registration Date : 2023-09-11
Registration Number : 20230911-210-J-1543
Manufacturer Name : Zhejiang Hichi Pharmaceutica...
Manufacturer Address : No.36 Changshun Road, Bingang Industrial Zone, Shamen Town, Yuhuan, Zhejiang, China

Registrant Name : Korea Pavise Pharmaceutical Co., Ltd.
Registration Date : 2019-10-30
Registration Number : 20180814-210-J-252(5)
Manufacturer Name : Zhejiang Taizhou Hisyn Pharm...
Manufacturer Address : Chemical and Medical Raw Materials Base Linhai Park, Zhejiang Province China

Registrant Name : Central Medical Service Co., Ltd.
Registration Date : 2024-09-26
Registration Number : 20210215-210-J-472(1)
Manufacturer Name : Zhejiang Haizhou Pharmaceuti...
Manufacturer Address : Yanhai Industrial Zone, Linhai City, Zhejiang Province, China

Registrant Name : Korea Biochem Pharmaceutical Co., Ltd.
Registration Date : 2022-04-27
Registration Number : 20210308-210-J-719(1)
Manufacturer Name : Zhejiang Starry Pharmaceutic...
Manufacturer Address : No.1 Starry Road, Xianju Modern Industrial Centralization Zone, Xianju County, Zhejia...

Registrant Name : Encomac Co., Ltd.
Registration Date : 2022-07-15
Registration Number : 20210308-210-J-719(2)
Manufacturer Name : Zhejiang Starry Pharmaceutic...
Manufacturer Address : No.1 Starry Road, Xianju Modern Industrial Centralization Zone, Xianju County, Zhejia...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]NDC Package Code : 62331-066
Start Marketing Date : 2022-07-04
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

NDC Package Code : 65072-0801
Start Marketing Date : 2020-07-10
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

NDC Package Code : 43228-100
Start Marketing Date : 1985-12-26
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Biophore is a research-driven global pharmaceutical company focused on niche APIs for the generic industry.
Biophore is a research-driven global pharmaceutical company focused on niche APIs for the generic industry.
About the Company : Biophore, founded in 2007, has established itself as a reliable partner in the development and manufacturing of niche and complex pharmaceutical products. With 4 USFDA and EU-appro...
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
About the Company : LGM Pharma is a global leader in sourcing hard-to-find APIs and intermediates for the pharmaceutical and biotech industries. LGM is also a full service CDMO providing formulation, ...
 Rochem, your partner in developing, sourcing, and supplying pharmaceutical & animal health ingredients of Chinese origin.
Rochem, your partner in developing, sourcing, and supplying pharmaceutical & animal health ingredients of Chinese origin.
About the Company : Established in 1994, Rochem is a distributor of pharmaceutical, food, nutritional and animal health ingredients to some of the largest companies in the world. It sources high-quali...
About the Company : Arde’s Laboratories Pvt Ltd. is a specialty pharmaceutical company that is engaged in the development, manufacture and marketing of generic pharmaceutical drugs. Arde’s is buil...

About the Company : Brother Enterprises Holding Co., Ltd. was founded in 1991 and is headquartered in Haining City, Zhejiang Province, China. With over 30 years of experience, Brother Enterprises has ...

About the Company : Divi's Laboratories Limited is a leading independent contract manufacturer of Active Pharmaceutical Ingredients and registered intermediates. We custom / exclusively manufacture fo...

About the Company : As an EU-GMP certified global company and an established hallmark for pharmaceutical standards, Global Calcium has stood the test of time since its inception in 1979 as Calcium Ind...

About the Company : The Juste Group is the result of a merger of two companies: Justesa Imagen, centered on Chemicals, and Juste Farma, a pharmaceutical company. It has completed 100 years of growth, ...

About the Company : HENGDIAN GROUP was established in 1975, till now it has become a transnational,conglomerate group and been one of the largest private-owned enterprises in China. The Pharmaceutical...

About the Company : Hetero is a research based global pharmaceutical company focused on development, manufacturing and marketing of Active Pharmaceutical Ingredients (APIs), Intermediate Chemicals & F...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
3 - Chloro 1,2 - propanediol (CPD)
CAS Number : CAS-96-24-2
End Use API : Iohexol
About The Company : Blue Jet Healthcare is a global, science-driven pharmaceutical company specializing in collaboration, development, and manufacturing of advanced pharmaceutical ...

CAS Number : CAS-616-30-8
End Use API : Iohexol
About The Company : Blue Jet Healthcare is a global, science-driven pharmaceutical company specializing in collaboration, development, and manufacturing of advanced pharmaceutical ...

5-Amino-N,N'- bis(2,3- Dihydroxypropyl)- 2,4,6- Tr...
CAS Number : CAS-76801-93-9
End Use API : Iohexol
About The Company : Blue Jet Healthcare is a global, science-driven pharmaceutical company specializing in collaboration, development, and manufacturing of advanced pharmaceutical ...

CAS Number : CAS-618-88-2
End Use API : Iohexol
About The Company : Blue Jet Healthcare is a global, science-driven pharmaceutical company specializing in collaboration, development, and manufacturing of advanced pharmaceutical ...

5-Nitroisophthalic Acid Dimethyl Ester (NIPA-DME)
CAS Number : CAS-13290-96-5
End Use API : Iohexol
About The Company : Blue Jet Healthcare is a global, science-driven pharmaceutical company specializing in collaboration, development, and manufacturing of advanced pharmaceutical ...

CAS Number : CAS-618-88-2
End Use API : Iohexol
About The Company : Shreeneel Chemicals is dedicated to quality healthcare development and fostering a knowledgeable environment. The company was founded by Mr. Sanjay Tavethiya an...

CAS Number : CAS-13290-96-5
End Use API : Iohexol
About The Company : Shreeneel Chemicals is dedicated to quality healthcare development and fostering a knowledgeable environment. The company was founded by Mr. Sanjay Tavethiya an...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : OMNIPAQUE 180
Dosage Form : SOLUTION;INJECTION, ORAL, RECTAL
Dosage Strength : 38.8%
Approval Date : 1985-12-26
Application Number : 18956
RX/OTC/DISCN : RX
RLD : Yes
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : OMNIPAQUE 240
Dosage Form : SOLUTION;INJECTION, ORAL, RECTAL
Dosage Strength : 51.8%
Approval Date : 1985-12-26
Application Number : 18956
RX/OTC/DISCN : RX
RLD : Yes
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : OMNIPAQUE 300
Dosage Form : SOLUTION;INJECTION, ORAL, RECTAL
Dosage Strength : 64.7%
Approval Date : 1985-12-26
Application Number : 18956
RX/OTC/DISCN : RX
RLD : Yes
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : OMNIPAQUE 350
Dosage Form : SOLUTION;INJECTION, ORAL
Dosage Strength : 75.5%
Approval Date : 1985-12-26
Application Number : 18956
RX/OTC/DISCN : RX
RLD : Yes
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : OMNIPAQUE 140
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : 30.2%
Approval Date : 1988-11-30
Application Number : 18956
RX/OTC/DISCN : RX
RLD : Yes
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : OMNIPAQUE 210
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : 45.3%
Approval Date : 1989-06-30
Application Number : 18956
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : OMNIPAQUE 70
Dosage Form : SOLUTION;URETHRAL
Dosage Strength : 15.1%
Approval Date : 1994-06-01
Application Number : 18956
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : OMNIPAQUE 240
Dosage Form : SOLUTION;INJECTION, ORAL, RECTAL
Dosage Strength : 51.8%
Approval Date : 1995-10-24
Application Number : 20608
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : OMNIPAQUE 300
Dosage Form : SOLUTION;INJECTION, ORAL, RECTAL
Dosage Strength : 64.7%
Approval Date : 1995-10-24
Application Number : 20608
RX/OTC/DISCN : RX
RLD : No
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : OMNIPAQUE 350
Dosage Form : SOLUTION;INJECTION, ORAL
Dosage Strength : 75.5%
Approval Date : 1995-10-24
Application Number : 20608
RX/OTC/DISCN : RX
RLD : No
TE Code :

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Originator
Registration Country : South Africa
Brand Name : Omnipaque 300Mg I/Ml (200Ml)
Dosage Form : INJ
Dosage Strength : 300mg/ml
Packaging : 200X1mg/ml
Approval Date :
Application Number :
Regulatory Info : Originator
Registration Country : South Africa

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Originator
Registration Country : South Africa
Brand Name : Omnipaque 300Mg I/Ml (500Ml)
Dosage Form : Inj
Dosage Strength : 300mg/ml
Packaging : 500X1mg/ml
Approval Date :
Application Number :
Regulatory Info : Originator
Registration Country : South Africa

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Originator
Registration Country : South Africa
Brand Name : Omnipaque 350Mg I/Ml (200Ml)
Dosage Form : Inj
Dosage Strength : 350mg/ml
Packaging : 200X1mg/ml
Approval Date :
Application Number :
Regulatory Info : Originator
Registration Country : South Africa

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Originator
Registration Country : South Africa
Brand Name : Omnipaque 350Mg I/Ml (500Ml)
Dosage Form : Inj
Dosage Strength : 350mg/ml
Packaging : 500X1mg/ml
Approval Date :
Application Number :
Regulatory Info : Originator
Registration Country : South Africa

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Originator
Registration Country : South Africa
Brand Name : Omnipaque 300Mg I/Ml (100Ml)
Dosage Form : Inj
Dosage Strength : 300mg/ml
Packaging : 100X1mg/ml
Approval Date :
Application Number :
Regulatory Info : Originator
Registration Country : South Africa

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Originator
Registration Country : South Africa
Brand Name : Omnipaque 300Mg I/Ml (20Ml)
Dosage Form : Inj
Dosage Strength : 300mg/ml
Packaging : 20X1mg/ml
Approval Date :
Application Number :
Regulatory Info : Originator
Registration Country : South Africa

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Originator
Registration Country : South Africa
Brand Name : Omnipaque 300Mg I/Ml (50Ml)
Dosage Form : Inj
Dosage Strength : 300mg/ml
Packaging : 50X1mg/ml
Approval Date :
Application Number :
Regulatory Info : Originator
Registration Country : South Africa

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Originator
Registration Country : South Africa
Brand Name : Omnipaque 350Mg I/Ml (50Ml)
Dosage Form : Inj
Dosage Strength : 350mg/ml
Packaging : 50X1mg/ml
Approval Date :
Application Number :
Regulatory Info : Originator
Registration Country : South Africa

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Originator
Registration Country : South Africa
Brand Name : Omnipaque 350Mg I/Ml (100Ml)
Dosage Form : Inj
Dosage Strength : 350mg/ml
Packaging : 100X1mg/ml
Approval Date :
Application Number :
Regulatory Info : Originator
Registration Country : South Africa

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Biophore is a research-driven global pharmaceutical company focused on niche APIs for the generic industry.
Biophore is a research-driven global pharmaceutical company focused on niche APIs for the generic industry.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Injection
Dosage Strength : 240MG/ML
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
 Biophore is a research-driven global pharmaceutical company focused on niche APIs for the generic industry.
Biophore is a research-driven global pharmaceutical company focused on niche APIs for the generic industry.
Packaging :
Regulatory Info :
Dosage : Injection
Dosage Strength : 240MG/ML
Brand Name :
Approval Date :
Application Number :
Registration Country : India
 Biophore is a research-driven global pharmaceutical company focused on niche APIs for the generic industry.
Biophore is a research-driven global pharmaceutical company focused on niche APIs for the generic industry.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Injection
Dosage Strength : 300MG/ML
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
 Biophore is a research-driven global pharmaceutical company focused on niche APIs for the generic industry.
Biophore is a research-driven global pharmaceutical company focused on niche APIs for the generic industry.
Packaging :
Regulatory Info :
Dosage : Injection
Dosage Strength : 300MG/ML
Brand Name :
Approval Date :
Application Number :
Registration Country : India
 Biophore is a research-driven global pharmaceutical company focused on niche APIs for the generic industry.
Biophore is a research-driven global pharmaceutical company focused on niche APIs for the generic industry.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Injection
Dosage Strength : 350MG/ML
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
 Biophore is a research-driven global pharmaceutical company focused on niche APIs for the generic industry.
Biophore is a research-driven global pharmaceutical company focused on niche APIs for the generic industry.
Packaging :
Regulatory Info :
Dosage : Injection
Dosage Strength : 350MG/ML
Brand Name :
Approval Date :
Application Number :
Registration Country : India
 Naprod is a top player in Oncology & Anesthesia FDF through excellent innovation, discovery of new processes & high-quality production.
Naprod is a top player in Oncology & Anesthesia FDF through excellent innovation, discovery of new processes & high-quality production.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Liquid Injection
Dosage Strength : 755MG/ML
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
 Naprod is a top player in Oncology & Anesthesia FDF through excellent innovation, discovery of new processes & high-quality production.
Naprod is a top player in Oncology & Anesthesia FDF through excellent innovation, discovery of new processes & high-quality production.
Packaging :
Regulatory Info :
Dosage : Liquid Injection
Dosage Strength : 755MG/ML
Brand Name :
Approval Date :
Application Number :
Registration Country : India
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : China
Brand Name :
Dosage Form : Injection
Dosage Strength : 51.8%
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : China

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dosage : Injection
Dosage Strength : 51.8%
Brand Name :
Approval Date :
Application Number :
Registration Country : China

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : China
Brand Name :
Dosage Form : Injection
Dosage Strength : 64.7%
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : China

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dosage : Injection
Dosage Strength : 64.7%
Brand Name :
Approval Date :
Application Number :
Registration Country : China

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : China
Brand Name :
Dosage Form : Injection
Dosage Strength : 75.5%
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : China

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dosage : Injection
Dosage Strength : 75.5%
Brand Name :
Approval Date :
Application Number :
Registration Country : China

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : India
Brand Name :
Dosage Form : Injection
Dosage Strength : 240MGI/1ML
Packaging :
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info : Generic
Dosage : Injection
Dosage Strength : 240MGI/1ML
Brand Name :
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : India
Brand Name :
Dosage Form : Injection
Dosage Strength : 300MGI/1ML
Packaging :
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info : Generic
Dosage : Injection
Dosage Strength : 300MGI/1ML
Brand Name :
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : China
Brand Name :
Dosage Form : Injection
Dosage Strength : 30G
Packaging : 100mL:30g(I)
Approval Date :
Application Number :
Regulatory Info :
Registration Country : China

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging : 100mL:30g(I)
Regulatory Info :
Dosage : Injection
Dosage Strength : 30G
Brand Name :
Approval Date :
Application Number :
Registration Country : China

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Reply
07 Oct 2024
Reply
31 Jul 2024
Reply
17 Jun 2023
Reply
27 Sep 2022
Reply
21 Jun 2022
Reply
13 Apr 2022
Reply
12 Apr 2022
Reply
29 Jan 2022
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?