
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
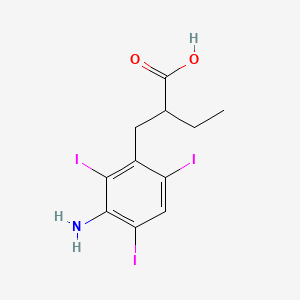

1. Acid, Iodopanoic
2. Acid, Iopanoic
3. Cholevid
4. Iodopanoic Acid
5. Iopagnost
6. Polognost
7. Telepaque
1. 96-83-3
2. Iodopanoic Acid
3. Telepaque
4. Cholevid
5. Iopagnost
6. Iopanic Acid
7. Bilijodon
8. Choladine
9. Cistobil
10. Copanoic
11. Jopagnost
12. Polognost
13. Colepax
14. Iopanoicum
15. Jopanoic Acid
16. 2-(3-amino-2,4,6-triiodobenzyl)butyric Acid
17. 2-(3-amino-2,4,6-triiodobenzyl)butanoic Acid
18. Acidum Iopanoicum
19. Teletrast
20. Iodopanic Acid
21. 2-[(3-amino-2,4,6-triiodophenyl)methyl]butanoic Acid
22. Nsc 41706
23. Iopanoate
24. 3-amino-alpha-ethyl-2,4,6-triiodohydrocinnamic Acid
25. Nsc-41706
26. 2-ethyl-3-(3-amino-2,4,6-triiodophenyl)propionic Acid
27. 3-(3-amino-2,4,6-triiodophenyl)-2-ethylpropanoic Acid
28. 3-amino-alpha-ethyl-2,4,6-triiodobenzenepropanoic Acid
29. Chebi:5951
30. Fe9794p71j
31. 2-[(3-amino-2,4,6-triiodo-phenyl)methyl]butanoic Acid
32. Benzenepropanoic Acid, 3-amino-.alpha.-ethyl-2,4,6-triiodo-
33. Dsstox_cid_3159
34. Dsstox_rid_76897
35. Dsstox_gsid_23159
36. 3-amino-.alpha.-ethyl-2,4,6-triiodobenzenepropanoic Acid
37. Hydrocinnamic Acid, 3-amino-.alpha.-ethyl-2,4,6-triiodo-
38. Acido Iopanoico
39. Acide Iopanoique
40. .alpha.-ethyl-.beta.-(3-amino-2,4,6-triiodophenyl)propionic Acid
41. Acide Iopanoique [french]
42. Acide Iopanoique [inn-french]
43. Acido Iopanoico [inn-spanish]
44. Acidum Iopanoicum [inn-latin]
45. Acido Iopanoico [latin,spanish]
46. Hsdb 3345
47. Sr-05000001877
48. Win 2011
49. Einecs 202-539-9
50. Brn 2220381
51. Unii-fe9794p71j
52. Benzenepropanoic Acid, 3-amino-alpha-ethyl-2,4,6-triiodo-
53. Cas-96-83-3
54. Telepaque (tn)
55. Ncgc00016357-01
56. Iopanoic Acid [usp:inn:ban:jan]
57. Hydrocinnamic Acid, 3-amino-alpha-ethyl-2,4,6-triiodo-
58. Spectrum_001576
59. Beta-(3-amino-2,4,6-triiodophenyl)-alpha-ethylpropionic Acid
60. Prestwick0_001052
61. Prestwick1_001052
62. Prestwick2_001052
63. Prestwick3_001052
64. Spectrum2_001206
65. Spectrum4_000879
66. Spectrum5_001681
67. Chembl867
68. Iopanoic Acid [mi]
69. (.+/-.)-iopanoic Acid
70. Iopanoic Acid [inn]
71. Iopanoic Acid [jan]
72. Schembl38976
73. Bspbio_001004
74. Iopanoic Acid [hsdb]
75. Kbiogr_001577
76. Kbioss_002056
77. Mls002154127
78. Divk1c_000902
79. Iopanoic Acid [vandf]
80. Spectrum1503923
81. Spbio_000992
82. Spbio_002932
83. Iopanoic Acid [mart.]
84. Bpbio1_001106
85. Iopanoic Acid (jan/usp/inn)
86. Iopanoic Acid [who-dd]
87. Iopanoic Acid [who-ip]
88. Dtxsid6023159
89. Hms502n04
90. Kbio1_000902
91. Kbio2_002056
92. Kbio2_004624
93. Kbio2_007192
94. Oirfjrbsrorbcm-uhfffaoysa-
95. (pm)-3-amino-alpha-ethyl-2,4,6-triiodohydrocinnamic Acid
96. Hydrocinnamic Acid,4,6-triiodo-
97. Ninds_000902
98. Hms1571c06
99. Hms1922k20
100. Hms2093m03
101. Hms2098c06
102. Hms2231g12
103. Hms3369d07
104. Hms3715c06
105. Iopanoic Acid, Analytical Standard
106. Pharmakon1600-01503923
107. Benzenepropanoic Acid,4,6-triiodo-
108. Hy-b1664
109. Iopanoic Acid [ep Impurity]
110. Iopanoic Acid [orange Book]
111. Nsc41706
112. Tox21_110394
113. Tox21_303477
114. Benzenepropanoic Acid, 3-amino-alpha-ethyl-2,4,6-triiodo-, (+-)-
115. Ccg-39139
116. Iopanoic Acid [ep Monograph]
117. Iopanoic Acid [usp Impurity]
118. Mfcd00038687
119. Nsc758646
120. S5497
121. Akos015854598
122. Tox21_110394_1
123. Cs-8125
124. Db08946
125. Nsc-758646
126. Idi1_000902
127. Wln: Zr Bi Di Fi C1y2 & Vq
128. Acidum Iopanoicum [who-ip Latin]
129. Ncgc00095082-01
130. Ncgc00095082-02
131. Ncgc00095082-03
132. Ncgc00095082-06
133. Ncgc00257386-01
134. As-66216
135. Smr001233434
136. Sbi-0051863.p002
137. 2-(2-carbamimidoylsulfanylethyl)isothiourea
138. 2-(3-amino-2,6-triiodobenzyl)butyric Acid
139. Ab00052387
140. Ft-0631496
141. I0300
142. C08217
143. D01014
144. T73105
145. Ab00052387_07
146. 2-(3-amino-2,4,6-triiodobenzyl)butanoic Acid #
147. A845646
148. Q1672108
149. Sr-05000001877-1
150. Sr-05000001877-3
151. W-100130
152. 2-ethyl-3-(3-amino-2,6-triiodophenyl)propionic Acid
153. 3-(3-amino-2,6-triiodophenyl)-2-ethylpropanoic Acid
154. 3-amino-.alpha.-ethyl-2,6-triiodohydrocinnamic Acid
155. 3-amino-alpha-ethyl-2,4,6-triiodobenzenepropanoicacid
156. Brd-a42628519-001-04-1
157. Brd-a42628519-001-07-4
158. 3-(3-amino-2,4,6-triiodophenyl)-2-ethylpropionic Acid
159. 3-amino-.alpha.-ethyl-2,4,6-triiodohydrocinnamic Acid
160. Iopanoic Acid, European Pharmacopoeia (ep) Reference Standard
161. (pm)-3-amino-.alpha.-ethyl-2,4,6-triiodohydrocinnamic Acid
162. .beta.-(3-amino-2,4,6-triiodophenyl)-.alpha.-ethylpropionic Acid
163. .beta.-(3-amino-2,6-triiodophenyl)-.alpha.-ethylpropionic Acid
164. Benzenepropanoic Acid, 3-amino-.alpha.-ethyl-2,4,6-triiodo-, (.+/-.)-
165. Benzenepropanoic Acid, 3-amino-.alpha.-ethyl-2,4,6-triiodo-, (+/-)-
| Molecular Weight | 570.93 g/mol |
|---|---|
| Molecular Formula | C11H12I3NO2 |
| XLogP3 | 3.9 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 4 |
| Exact Mass | 570.8002 g/mol |
| Monoisotopic Mass | 570.8002 g/mol |
| Topological Polar Surface Area | 63.3 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 277 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Contrast Media
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
ORALLY AS A RADIOPAQUE MEDIUM IN CHOLECYSTOGRAPHY. ALTHOUGH NOT THE METHOD OF CHOICE, IT MAY ALSO BE USED FOR ORAL CHOLANGIOGRAPHY.
Osol, A. (ed.). Remington's Pharmaceutical Sciences. 16th ed. Easton, Pennsylvania: Mack Publishing Co., 1980., p. 1213
USUAL REGIMEN IS TO GIVE PT FAT-FREE EVENING MEAL FOLLOWING WHICH IOPANOIC ACID IS ADMIN APPROX 10 HR BEFORE TIME SCHEDULED FOR ROENTGENOGRAPHY. IMMEDIATELY AFTER ROENTGEN EXAM, PT IS GIVEN HIGH-FAT MEAL & ADDITIONAL EXPOSURES ARE MADE...TO EVALUATE CONTRACTION OF GALL BLADDER & TO VISUALIZE PATENCY OF EXTRAHEPATIC DUCTS. WHEN LATTER STRUCTURES ARE OF PARTICULAR INTEREST, DOSE OF IOPANOIC ACID MAY BE INCR TO 5 OR 6 G. DOSE--3 TO 6 G; USUAL, 3 G.
Osol, A. (ed.). Remington's Pharmaceutical Sciences. 16th ed. Easton, Pennsylvania: Mack Publishing Co., 1980., p. 1214
TYROPANOATE 3 G, IOCETAMIC ACID 4.5 G, IOPANOIC ACID 3 G & IOCETAMIC ACID 3 G, WERE EVALUATED IN 800 PT. IOCETAMIC ACID IS AS RELIABLE & EFFECTIVE AS TYROPANOATE OR IOPANOIC ACID IN CHOLECYSTOGRAPHY. 3 G DOSE PRODUCES CHOLECYSTOGRAMS AS SATISFACTORY AS 4.5 G WITH LOWER INCIDENCE OF CRAMPS.
PARKS R; DOUBLE-BLIND STUDY OF 4 ORAL CHOLECYSTOGRAPHIC PREPARATIONS; RADIOLOGY 112(SEP) 525 (1974)
IT IS CONTRAINDICATED IN PT WITH ACUTE NEPHRITIS AND UREMIA, SINCE IT IS ELIMINATED BY THE KIDNEYS. IT SHOULD NOT BE ADMIN WHEN DISORDERS OF THE GI TRACT EXIST WHICH PREVENT ABSORPTION OF THE MEDIUM.
Osol, A. (ed.). Remington's Pharmaceutical Sciences. 16th ed. Easton, Pennsylvania: Mack Publishing Co., 1980., p. 1214
IOPANOIC ACID APPEARS TO COMPETE WITH BILIRUBIN FOR HEPATIC UPTAKE, RESULTING IN TRANSIENT ELEVATIONS OF SERUM BILIRUBIN.
Hansten, P.D. Drug Interactions. 4th ed. Philadelphia: Lea and Febiger, 1979., p. 330
CONTRAST NEPHROPATHY IS AN ADVERSE ALTERATION IN RENAL FUNCTION INDUCED BY INTRAVASCULAR CONTRAST MEDIA. THE INCIDENCE OF CONTRAST NEPHROPATHY IN THE GENERAL HOSPITALIZED POPULATION IS ABOUT 5%, & IS ASSOCIATED WITH PREEXISTING RENAL INSUFFICIENCY & DIABETES MELLITUS. AS MANY AS TWO-THIRDS OF PATIENTS WITH CHRONIC RENAL FAILURE MAY EXPERIENCE AN ACUTE DETERIORATION IN RENAL FUNCTION FOLLOWING EXPOSURE. DIABETIC PATIENTS WITH PREEXISTING RENAL INSUFFICIENCY ARE AT AN EVEN GREATER RISK; ABOUT 75% OF SUCH PATIENTS WILL EXPERIENCE RENAL COMPLICATIONS. IN MULTIPLE MYELOMA THE RISK OF CONTRAST-INDUCED RENAL FAILURE IS LOW, & PROBABLY INVOLVES A DIFFERENT PATHOGENESIS THAN SEEN IN OTHER CASES OF CONTRAST NEPHROPATHY. PERIPHERAL VASCULAR DISEASE, HYPERTENSION, OLD AGE & LARGE & REPEATED DOSES OF CONTRAST MAY INCREASE THE RISK IN SUSCEPTIBLE PATIENTS.
HARKONEN S, KIELLSTRAND C; CONTRAST NEPHROPATHY; AM J NEPHROL 1 (2): 69-77 (1981)
Contrast Media
Substances used to allow enhanced visualization of tissues. (See all compounds classified as Contrast Media.)
V - Various
V08 - Contrast media
V08A - X-ray contrast media, iodinated
V08AC - Watersoluble, hepatotropic x-ray contrast media
V08AC06 - Iopanoic acid
AFTER INGESTION, IT IS ABSORBED PROMPTLY, UNDERGOES CONJUGATION WITH GLUCURONIC ACID IN LIVER, IS CONCENTRATED & STORED IN GALLBLADDER, & IS ELIMINATED IN BILE.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 802
FAILURE OF IOPANOIC ACID TO REACH DIAGNOSTIC CONCN IN BILE AFTER.../ORAL/ DOSES HAS BEEN SHOWN TO BE DUE, IN PART @ LEAST, TO IRREGULAR ABSORPTION. ABSORPTION OF.../ORAL/ DOSED IOPANOIC ACID IN DOGS WAS INCR IN PRESENCE OF BILE SALTS & SODIUM SALT FORM OF THIS AGENT IS ABSORBED MORE UNIFORMLY THAN FREE ACID.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 4: A Review of the Literature Published during 1974 and 1975. London: The Chemical Society, 1977., p. 75
BILIARY EXCRETION RATE OF SODIUM IOPANOATE FITTED BY COMPUTER TO MICHAELIS-MENTEN EQUATION AGAINST ITS UNBOUND PLASMA CONCN AVG MAX VALUE 0.85 MU MOLAR/KG/MIN, & KM VALUE 0.253 MU MOLAR. UNCHANGED IN MONKEY BLOOD & MAINLY NA IOPANOATE ESTER GLUCURONIDE IN BILE.
MOSS AA ET AL; PHARMACOKINETICS OF IOPANOIC ACID IN THE RHESUS MONKEY; BILIARY EXCRETION, PLASMA PROTEIN BINDING AND BIOTRANSFORMATION; INVEST RADIOL 14(2) 171 (1979)
MAX EXCRETION OF IOPANOIC ACID INTO BILE OF UNANESTHETIZED DOGS WITH BILE FISTULA WAS CLOSELY CORRELATED TO EXCRETION RATE OF BILE SALTS.
LOEB PM ET AL; DEPENDENCE OF THE BILIARY EXCRETION OF IOPANOIC ACID ON BILE SALTS; GASTROENTEROLOGY 72(2) 174 (1978)
For more Absorption, Distribution and Excretion (Complete) data for IOPANOIC ACID (6 total), please visit the HSDB record page.
...IOPANOIC ACID...& TYROPANOIC ACID...IN DOGS & MAN...IDENTIFIED AS ESTER GLUCURONIDES. ... POORLY ABSORBED IN CATS... IOPANOIC ACID...PREVIOUSLY... FOUND...TRANSFORMED INTO...WATER-SOL CONJUGATE IN CATS & RE-EXAMINATION OF PROBLEM ESTABLISHED LIMITED GLUCURONIC ACID CONJUGATION OF IOPANOIC & TYROPANOIC ACIDS.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 1: A Review of the Literature Published Between 1960 and 1969. London: The Chemical Society, 1970., p. 423
.../IT/ SUPPRESSES THYROID FUNCTION IN EUTHYROID AND HYPERTHYROID INDIVIDUALS.
American Medical Association, AMA Department of Drugs. AMA Drug Evaluations. 5th ed. Chicago: American Medical Association, 1983., p. 1068
TSH INDUCED SECRETION OF 3,3'-DIIODOTHYRONINE, 3',5'-T2, & 3,5-T2 BY PERFUSED DOG THYROID WAS DETERMINED. IOPANOATE (10-5 MOLAR) & IPODATE (10-5 MOLAR), BOTH INHIBITORS OF PERIPHERAL IODOTHYRONINE DEIODINATION, INHIBITED 3,3'-DIIODOTHYRONINE SECRETION.
LAURBERG P; SECRETION OF DIIODOTHYRONINES FROM THE PERFUSED CANINE THYROID ISOLATED IN SITU; THE INFLUENCE OF RADIOCONTRAST AGENTS; THYROID RES 8 (8) (EIGHT) PROC INT THYROID CONGR 8TH 359-62 (1980)
THE ABILITY OF ROENTGENOGRAPHIC CONTRAST AGENTS TO INHIBIT BINDING OF (125)I-LABELED T3 TO NUCLEAR RECEPTORS WAS STUDIED DURING INCUBATION OF RAT LIVER NUCLEI OR NUCLEAR EXTRACTS IN VITRO & AFTER IP ADMIN OF THE AGENTS IN VIVO. IOPANOIC ACID INHIBITED BINDING OF T3-(125)I IN VITRO.
DEGROOT LJ, RUE PA; ROENTGENOGRAPHIC CONTRAST AGENTS INHIBIT TRIIODOTHYRONINE BINDING TO NUCLEAR RECEPTORS IN VITRO; J CLIN ENDOCRINOL METAB 49 (4): 538-42 (1979)
CHANGES IN CONCN OF TSH, THYROXINE, T3, & REVERSE T3 IN SERUM WERE EXAMINED IN 21 MALE EUTHYROID SUBJECTS AFTER ORAL ADMIN OF 3 G/DAY OF IOPANOIC ACID. CONCN OF TSH, THYROXINE, & REVERSE T3 INCREASED, WHEREAS CONCN OF T3 DECREASED AFTER ADMIN OF IOPANOIC ACID.
SUZUKI H ET AL; EFFECTS OF ORAL CHOLECYSTOGRAPHIC AGENTS ON THE PITUITARY-THYROID AXIS. I. CHANGES IN SERUM IODOTHYRONINES AND THYROTROPIN AFTER REPEATED DOSES OF ORAL CHOLECYSTOGRAPHIC AGENTS AND COMPARISON OF THE EFFECTS AMONG SOME IODOCONTRAST AGENTS; SAISHIN IGAKU 35 (1): 194-8 (1980)
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
78
PharmaCompass offers a list of Iopanoic Acid API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Iopanoic Acid manufacturer or Iopanoic Acid supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Iopanoic Acid manufacturer or Iopanoic Acid supplier.
PharmaCompass also assists you with knowing the Iopanoic Acid API Price utilized in the formulation of products. Iopanoic Acid API Price is not always fixed or binding as the Iopanoic Acid Price is obtained through a variety of data sources. The Iopanoic Acid Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Iopanoic Acid manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Iopanoic Acid, including repackagers and relabelers. The FDA regulates Iopanoic Acid manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Iopanoic Acid API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Iopanoic Acid supplier is an individual or a company that provides Iopanoic Acid active pharmaceutical ingredient (API) or Iopanoic Acid finished formulations upon request. The Iopanoic Acid suppliers may include Iopanoic Acid API manufacturers, exporters, distributors and traders.
click here to find a list of Iopanoic Acid suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Iopanoic Acid DMF (Drug Master File) is a document detailing the whole manufacturing process of Iopanoic Acid active pharmaceutical ingredient (API) in detail. Different forms of Iopanoic Acid DMFs exist exist since differing nations have different regulations, such as Iopanoic Acid USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Iopanoic Acid DMF submitted to regulatory agencies in the US is known as a USDMF. Iopanoic Acid USDMF includes data on Iopanoic Acid's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Iopanoic Acid USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Iopanoic Acid suppliers with USDMF on PharmaCompass.
Iopanoic Acid Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Iopanoic Acid GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Iopanoic Acid GMP manufacturer or Iopanoic Acid GMP API supplier for your needs.
A Iopanoic Acid CoA (Certificate of Analysis) is a formal document that attests to Iopanoic Acid's compliance with Iopanoic Acid specifications and serves as a tool for batch-level quality control.
Iopanoic Acid CoA mostly includes findings from lab analyses of a specific batch. For each Iopanoic Acid CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Iopanoic Acid may be tested according to a variety of international standards, such as European Pharmacopoeia (Iopanoic Acid EP), Iopanoic Acid JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Iopanoic Acid USP).
