

Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
API
0
FDF
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
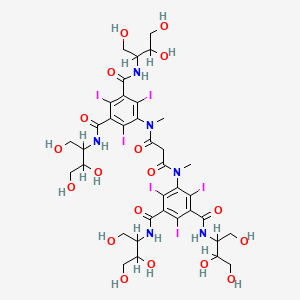

1. Dl 3-117
2. Dl 3117
3. Dl-3-117
4. Iotrol
5. Isovist
6. Isovist 300
7. Osmovist
1. Iotrol
2. 79770-24-4
3. Isovist
4. Osmovist
5. Isovist 300
6. Dl-3117
7. Compound Zk 39482
8. Zk 39 482
9. 1,3-benzenedicarboxamide, 5,5'-((1,3-dioxo-1,3-propanediyl)bis(methylimino))bis(n,n'-bis(2,3-dihydroxy-1-(hydroxymethyl)propyl)-2,4,6-triiodo-
10. Iotrovist
11. 16fl47b687
12. 2,4,6-triiodo-5-[methyl-[3-oxo-3-[2,4,6-triiodo-n-methyl-3,5-bis(1,3,4-trihydroxybutan-2-ylcarbamoyl)anilino]propanoyl]amino]-1-n,3-n-bis(1,3,4-trihydroxybutan-2-yl)benzene-1,3-dicarboxamide
13. 5,5'-(malonylbis(methylimino))bis(n,n'-bis(2,3-dihydroxy-1-(hydroxymethyl)propyl)-2,4,6-triiodoisophthalamide)
14. Zk-39482
15. Zk-39-482
16. Iotrolanum [latin]
17. Osmovist 190
18. Osmovist 240
19. Iotrolanum
20. Iotrolum
21. Iotrolum [inn-latin]
22. Dl 3-117
23. Sh 437
24. Zk 39482
25. Unii-16fl47b687
26. Iotrolan(isovist)
27. Iotrolan [usan:inn:ban:jan]
28. Iotrolan [usan]
29. Iotrolan [inn]
30. Iotrolan [jan]
31. Iotrolan [mi]
32. Iotrolan [vandf]
33. Isovist 300 (tn)
34. Iotrolan [mart.]
35. Iotrolan [who-dd]
36. Schembl25535
37. Iotrolan (jan/usan/inn)
38. Iotrolan [orange Book]
39. Iotrolan For System Suitability
40. Iotrolan [ep Monograph]
41. Chembl1200555
42. Dtxsid0023165
43. Chebi:31715
44. Sh-l-437d
45. Db09487
46. Ft-0754152
47. D01714
48. 770i244
49. Q6064149
50. 5,5'-[(1,3-dioxopropane-1,3-diyl)bis(methylimino)]bis{n,n'-bis[2,3-dihydroxy-1-(hydroxymethyl)propyl]-2,4,6-triiodobenzene-1,3-dicarboxamide}
| Molecular Weight | 1626.2 g/mol |
|---|---|
| Molecular Formula | C37H48I6N6O18 |
| XLogP3 | -4.2 |
| Hydrogen Bond Donor Count | 16 |
| Hydrogen Bond Acceptor Count | 18 |
| Rotatable Bond Count | 24 |
| Exact Mass | 1625.7293 g/mol |
| Monoisotopic Mass | 1625.7293 g/mol |
| Topological Polar Surface Area | 400 Ų |
| Heavy Atom Count | 67 |
| Formal Charge | 0 |
| Complexity | 1450 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 8 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Contrast Media
Substances used to allow enhanced visualization of tissues. (See all compounds classified as Contrast Media.)
V - Various
V08 - Contrast media
V08A - X-ray contrast media, iodinated
V08AB - Watersoluble, nephrotropic, low osmolar x-ray contrast media
V08AB06 - Iotrolan
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 5103
Submission : 1983-08-23
Status : Inactive
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 5103
Submission : 1983-08-23
Status : Inactive
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : OSMOVIST 190
Dosage Form : INJECTABLE;INTRATHECAL
Dosage Strength : 40.6%
Approval Date : 1989-12-07
Application Number : 19580
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : OSMOVIST 240
Dosage Form : INJECTABLE;INTRATHECAL
Dosage Strength : 51.3%
Approval Date : 1989-12-07
Application Number : 19580
RX/OTC/DISCN : DISCN
RLD : No
TE Code :

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
