
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
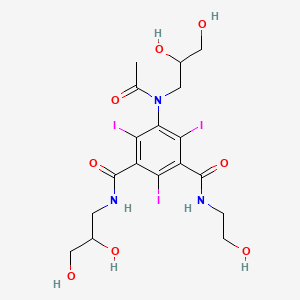

1. 5-(n-2,3-dihydroxypropylacetamido)-2,4,6-triiodo-n-(2-hydroxyethyl)-n'-(2,3-dihydroxypropyl)isophthalamide
2. Oxilan
1. 107793-72-6
2. Oxilan
3. Ioxitol
4. Oxilan-300
5. Nsc-760056
6. A4yj7j11tg
7. 5-[acetyl(2,3-dihydroxypropyl)amino]-3-n-(2,3-dihydroxypropyl)-1-n-(2-hydroxyethyl)-2,4,6-triiodobenzene-1,3-dicarboxamide
8. N-(2,3-dihydroxypropyl)-5-(n-(2,3-dihydroxypropyl)acetamido)-n'-(2-hydroxyethyl)-2,4,6-triiodoisophthalamide
9. Ncgc00182070-02
10. Dsstox_cid_28643
11. Dsstox_rid_82913
12. Dsstox_gsid_48717
13. Ioxilane
14. Ioxilanum
15. 1,3-benzenedicarboxamide, 5-(acetyl(2,3-dihydroxypropyl)amino)-n-(2,3-dihydroxypropyl)-n'-(2-hydroxyethyl)-2,4,6-triiodo-
16. Cas-107793-72-6
17. Oxilan-350
18. Ioxilane [inn-french]
19. Ioxilanum [inn-latin]
20. Unii-a4yj7j11tg
21. Ioxilan [usan:usp:inn]
22. Imagenil
23. Loxilan
24. Ccris 6727
25. 1,3-benzenedicarboxamide, 5-(acetyl(2,3-dihydroxypropyl)amino)-n1-(2,3-dihydroxypropyl)-n3-(2-hydroxyethyl)-2,4,6-triiodo-
26. 1,3-benzenedicarboxamide, 5-[acetyl(2,3-dihydroxypropyl)amino]-n1-(2,3-dihydroxypropyl)-n3-(2-hydroxyethyl)-2,4,6-triiodo-
27. Ioxilan(400 Mg)
28. Ioxilan [vandf]
29. Loxilan [vandf]
30. Ioxilan [usan]
31. Ioxilan [inn]
32. Ioxilan [jan]
33. Ioxilan [mi]
34. Oxilan-300 (tn)
35. Ioxilan [mart.]
36. Ioxilan [usp-rs]
37. Ioxilan [who-dd]
38. Ioxilan (jan/usp/inn)
39. Schembl25634
40. Ioxilan [orange Book]
41. Ioxilan (400 Mg)
42. Ioxilan [usp Impurity]
43. Ioxilan [usp Monograph]
44. Chembl1201075
45. Dtxsid0048717
46. Chebi:135884
47. Hms3264d15
48. Moli000985
49. Pharmakon1600-01503838
50. Tox21_113127
51. Nsc760056
52. Akos016014026
53. Tox21_113127_1
54. Ccg-213210
55. Db09135
56. Nsc 760056
57. Ncgc00182070-03
58. Hy-109513
59. Cs-0031230
60. Ft-0627286
61. D02161
62. Ab01563289_01
63. Sr-01000883963
64. J-002011
65. Q6064819
66. Sr-01000883963-1
67. 3-(3-methyl-4-oxo-thiazolidin-2-ylideneamino)-benzoicacid
68. 5-(n-2,3-dihydroxypropylacetamido)-2,4,6-triiodo-n-(2-hydroxyethyl)-n -(2,3-dihydroxypropyl)-isophthalamide
69. 5-[acetyl(2,3-dihydroxypropyl)amino]-n-(2,3-dihydroxypropyl)-n'-(2-hydroxyethyl)-2,4,6-triiodoisophthalamide
70. N-(2,3-dihdroxypropyl)-n -(2-hydroxyethyl)-5-[n-(2,3-dihydroxypropyl)acetamido]-2,4,6-triiodoiosphthalamide
71. N-(2,3-dihydroxypropyl)-n'-(2- Hydroxyethyl)-5-[n-(2,3-dihydroxypropyl) Acetamido]-2,4,6-triiodoisophthal-amide
| Molecular Weight | 791.1 g/mol |
|---|---|
| Molecular Formula | C18H24I3N3O8 |
| XLogP3 | -2.4 |
| Hydrogen Bond Donor Count | 7 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 11 |
| Exact Mass | 790.8698 g/mol |
| Monoisotopic Mass | 790.8698 g/mol |
| Topological Polar Surface Area | 180 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 647 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 2 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Oxilan-300 |
| Active Ingredient | Ioxilan |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 62% |
| Market Status | Prescription |
| Company | Guerbet |
| 2 of 4 | |
|---|---|
| Drug Name | Oxilan-350 |
| Active Ingredient | Ioxilan |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 73% |
| Market Status | Prescription |
| Company | Guerbet |
| 3 of 4 | |
|---|---|
| Drug Name | Oxilan-300 |
| Active Ingredient | Ioxilan |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 62% |
| Market Status | Prescription |
| Company | Guerbet |
| 4 of 4 | |
|---|---|
| Drug Name | Oxilan-350 |
| Active Ingredient | Ioxilan |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 73% |
| Market Status | Prescription |
| Company | Guerbet |
When administered intra-arterially, Ioxilan is indicated for the following diagnostic tests: cerebral arteriography (300 mgI/mL), coronary arteriography and left ventriculography (350 mgI/mL), visceral angiography(350 mgI/mL), aortography(350 mgI/mL), and peripheral arteriography(350 mgI/mL). When administered intravenously, Ioxilan is indicated for excretory urography and contrast enhanced computed tomographic (CECT) imaging of the head and body (300 and 350 mgI/mL).
FDA Label
As with other iodinated contrast agents the degree of contrast enhancement is directly related to the iodine content in the administered dose.
Contrast Media
Substances used to allow enhanced visualization of tissues. (See all compounds classified as Contrast Media.)
V - Various
V08 - Contrast media
V08A - X-ray contrast media, iodinated
V08AB - Watersoluble, nephrotropic, low osmolar x-ray contrast media
V08AB12 - Ioxilan
Absorption
Peak iodine plasma levels occur immediately following rapid intravenous injection. Iodine plasma levels fall rapidly within 5 to 10 minutes. This can be accounted for by the dilution in the vascular and extravascular fluid compartments.
Route of Elimination
The average amount of ioxilan excreted unchanged in urine at 24 hours represents 93.7% of the dose in young healthy subjects (21-27 years) after intravenous administration. This finding suggests that, compared to the renal excretion, biliary and/or gastrointestinal excretion are not important.
Volume of Distribution
Ioxilan is distributed mainly in the blood as suggested by the apparent volume of distribution (central compartment), 7.2 1.0 L in women and 10.0 2.4 L in men
Clearance
The total clearance values were 95.4 11.1 mLmin-1 and 101.0 14.7 mLmin-1 and the renal clearance values were 89.4 13.3 mLmin-1 and 94.9 16.6 mLmin-1 for women and men, respectively.
There is no evidence for metabolism.
An initial fast distribution phase with a half-life of 13.1 4.2 minutes (women) or 23.5 15.3 minutes (men) was followed by an elimination phase with a half-life of 102.0 16.9 minutes (women) and 137 35.4 minutes (men).
Intravascular injection results in opacification of vessels in the path of flow of the contrast medium, permitting radiographic visualization of the internal structures of the human body until significant hemodilution occurs.
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
21
PharmaCompass offers a list of Ioxilan API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Ioxilan manufacturer or Ioxilan supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Ioxilan manufacturer or Ioxilan supplier.
PharmaCompass also assists you with knowing the Ioxilan API Price utilized in the formulation of products. Ioxilan API Price is not always fixed or binding as the Ioxilan Price is obtained through a variety of data sources. The Ioxilan Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Ioxilan manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Ioxilan, including repackagers and relabelers. The FDA regulates Ioxilan manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Ioxilan API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Ioxilan supplier is an individual or a company that provides Ioxilan active pharmaceutical ingredient (API) or Ioxilan finished formulations upon request. The Ioxilan suppliers may include Ioxilan API manufacturers, exporters, distributors and traders.
click here to find a list of Ioxilan suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Ioxilan DMF (Drug Master File) is a document detailing the whole manufacturing process of Ioxilan active pharmaceutical ingredient (API) in detail. Different forms of Ioxilan DMFs exist exist since differing nations have different regulations, such as Ioxilan USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Ioxilan DMF submitted to regulatory agencies in the US is known as a USDMF. Ioxilan USDMF includes data on Ioxilan's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Ioxilan USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Ioxilan suppliers with USDMF on PharmaCompass.
Ioxilan Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Ioxilan GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Ioxilan GMP manufacturer or Ioxilan GMP API supplier for your needs.
A Ioxilan CoA (Certificate of Analysis) is a formal document that attests to Ioxilan's compliance with Ioxilan specifications and serves as a tool for batch-level quality control.
Ioxilan CoA mostly includes findings from lab analyses of a specific batch. For each Ioxilan CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Ioxilan may be tested according to a variety of international standards, such as European Pharmacopoeia (Ioxilan EP), Ioxilan JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Ioxilan USP).

