
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
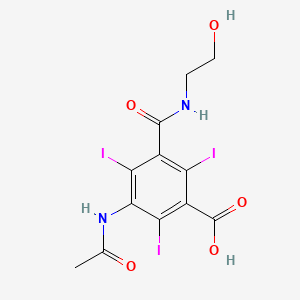

1. 5-acetamido-n-(2- Hydroxyethyl)-2,4,6-triiodoisophthalamic Acid
2. Agelix
3. Ioxitalamic Acid, Sodium Salt
4. Ioxithalamate
5. Sodium Ioxithalamate
6. Telebrix
7. Telebrix 38
8. Vasobrix
1. 28179-44-4
2. Acido Ioxitalamico
3. Acide Ioxitalamique
4. Acidum Ioxitalamicum
5. Iooxitalamic Acid
6. Ag 58107
7. Ioxithalamic Acid
8. 3-acetamido-5-(2-hydroxyethylcarbamoyl)-2,4,6-triiodobenzoic Acid
9. Telebrix
10. 3-acetamido-5-((2-hydroxyethyl)carbamoyl)-2,4,6-triiodobenzoic Acid
11. Ioxitalamic Acid Sodium Salt
12. 967rdi7z6k
13. Chebi:83517
14. Ag-58107
15. Ioxitalamic Acid (inn)
16. 3-(acetamido)-5-[[(2-hydroxyethyl)amino]carbonyl]-2,4,6-triiodo-benzoic Acid
17. Ioxitalamic Acid [inn]
18. Vasobrix
19. 5-acetamido-n-(2-hydroxyethyl)-2,4,6-triiodoisophthalamic Acid
20. 3-(acetylamino)-5-[[(2-hydroxyethyl)amino]carbonyl]-2,4,6-triiodobenzoic Acid
21. 3-(acetylamino)-5-{[(2-hydroxyethyl)amino]carbonyl}-2,4,6-triiodobenzoic Acid
22. Acido Iossitalamico
23. Acidum Joxitalamicum
24. Ioxitalamic Acid [inn:dcf]
25. Ioxitalamicacid
26. Acido Iossitalamico [dcit]
27. Unii-967rdi7z6k
28. 3-(acetylamino)-5-(((2-hydroxyethyl)amino)carbonyl)-2,4,6-triiodobenzoic Acid
29. Acide Ioxitalamique [inn-french]
30. Acido Ioxitalamico [inn-spanish]
31. Acidum Ioxitalamicum [inn-latin]
32. Einecs 248-887-5
33. Mls006010884
34. Schembl455411
35. Acido Ioxitalamico;ioxitalamate
36. Chembl2107239
37. Dtxsid60182457
38. Ioxitalamic Acid [mart.]
39. Ioxitalamic Acid [who-dd]
40. Act03777
41. Amy24792
42. Bcp34384
43. Zinc4216615
44. Mfcd00867942
45. Sodium 3-acetamido-5-(2-hydroxyethylcarbamoyl)-2,4,6-triiodobenzoate
46. Db13444
47. Smr004701800
48. Ft-0659353
49. D07418
50. F20665
51. 179i444
52. Sr-01000945067
53. Q3604532
54. Sr-01000945067-1
55. W-110718
56. 5-acetamido-2,4,6-triiodo-n-(2-hydroxyethyl)-isophthalamic Acid
57. 3-(acetylamino)-5-[(2-hydroxyethyl)carbamoyl]-2,4,6-triiodobenzoic Acid
58. 3-acetamido-2,4,6-triiodo-(n-beta-hydroxyethyl)isophthalic Acid Monoamide
| Molecular Weight | 643.94 g/mol |
|---|---|
| Molecular Formula | C12H11I3N2O5 |
| XLogP3 | 1.2 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 5 |
| Exact Mass | 643.7802 g/mol |
| Monoisotopic Mass | 643.7802 g/mol |
| Topological Polar Surface Area | 116 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 451 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Ioxitalamate in both of its available forms is indicated for exploration of the digestive tract by tomodensitometry or by regular gastroduodenal radiography. Its use is restrained to the cases in which the administration of barium sulfate is not recommended or contraindicated. The intravascular administration of ioxitalamate is contraindicated as it may present significant side effects.
Ioxitalamate presents a very large osmolality which is related to the presence of renal toxicity, vasodilatation, bradycardia and pulmonary hypertension. This large osmolality allows ioxitalamate to move slowly in the bowel allowing for analysis for later follow excretion in the feces.
Contrast Media
Substances used to allow enhanced visualization of tissues. (See all compounds classified as Contrast Media.)
V08AA05
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
V - Various
V08 - Contrast media
V08A - X-ray contrast media, iodinated
V08AA - Watersoluble, nephrotropic, high osmolar x-ray contrast media
V08AA05 - Ioxitalamic acid
Absorption
When administered ioxitalamate is not absorbed in the GI tract. In the case of presence of an intestinal perforation, ioxitalamate is completely absorbed. When administered intravascularly, ioxitalamate is rapidly distributed in the interstitial space and intravascular compartment.
Route of Elimination
As ioxitalamate is not absorbed in the normal intestine, the elimination route of this compound is entirely performed by the feces. When absorbed due to the presence of an intestinal perforation, ioxitalamate presents a rapid renal elimination. when ioxitalamate is administered intravascularly, it is eliminated unchanged mainly via renal excretion through glomerular filtration without reabsorption or tubular secretion. In the cases of renal failure, the elimination is mainly performed in the biliary, salivary, sudoral and colic route.
Volume of Distribution
The volume of distribution of ioxitalamate is 194 ml/kg.
Clearance
The total clearance rate of ioxitalamate is 120 ml/min.
The rapid clearance suggests that ioxitalamate is not metabolized in the body.
The elimination half-life of ioxitalamate is 1.1 hours.
Ioxitalamate acts as a bowel opacifier which facilitates the interpretation of the anatomy and differentiation of bowel loops from soft tissue masses.
Market Place

ABOUT THIS PAGE
78
PharmaCompass offers a list of Ioxitalamic Acid API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Ioxitalamic Acid manufacturer or Ioxitalamic Acid supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Ioxitalamic Acid manufacturer or Ioxitalamic Acid supplier.
PharmaCompass also assists you with knowing the Ioxitalamic Acid API Price utilized in the formulation of products. Ioxitalamic Acid API Price is not always fixed or binding as the Ioxitalamic Acid Price is obtained through a variety of data sources. The Ioxitalamic Acid Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Ioxitalamic Acid manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Ioxitalamic Acid, including repackagers and relabelers. The FDA regulates Ioxitalamic Acid manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Ioxitalamic Acid API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Ioxitalamic Acid manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Ioxitalamic Acid supplier is an individual or a company that provides Ioxitalamic Acid active pharmaceutical ingredient (API) or Ioxitalamic Acid finished formulations upon request. The Ioxitalamic Acid suppliers may include Ioxitalamic Acid API manufacturers, exporters, distributors and traders.
click here to find a list of Ioxitalamic Acid suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
Ioxitalamic Acid Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Ioxitalamic Acid GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Ioxitalamic Acid GMP manufacturer or Ioxitalamic Acid GMP API supplier for your needs.
A Ioxitalamic Acid CoA (Certificate of Analysis) is a formal document that attests to Ioxitalamic Acid's compliance with Ioxitalamic Acid specifications and serves as a tool for batch-level quality control.
Ioxitalamic Acid CoA mostly includes findings from lab analyses of a specific batch. For each Ioxitalamic Acid CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Ioxitalamic Acid may be tested according to a variety of international standards, such as European Pharmacopoeia (Ioxitalamic Acid EP), Ioxitalamic Acid JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Ioxitalamic Acid USP).
